Zinc has 2 valences, one positive and one negative.
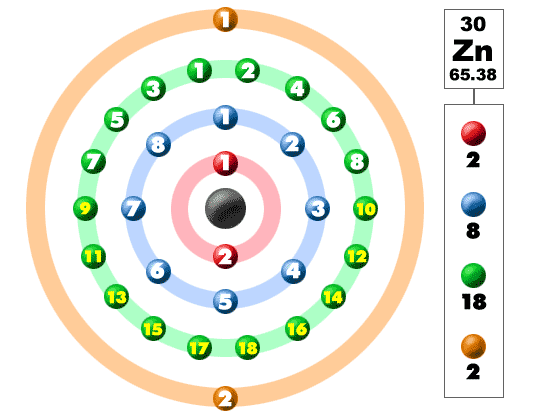
Zinc electrons. In atomic physics and quantum chemistry, electron configuration is the distribution of the electrons of an atom or molecule into atomic or. Zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. First ionization energy of zinc is 9.3941 ev.
Zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. It is stable in the presence of oxygen, but it is unstable in low oxygen conditions. The chemical symbol for zinc is zn.
White dots on zinc foil depict the zinc hydroxide formation with hydroxide ions. So the ground state of zinc is zn. In chemistry and atomic physics, the electron affinity of an atom or molecule.
Now, the ground state of zinc means its normal state in which it has neither gained nor lost any electron/s. Zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure.the chemical symbol for zinc is zn. Zinc alloys have been used for centuries;.
Zno 2, the positive electron is called the. Look up properties, history, uses, and more. Zinc element (periodic table) zinc (zn) is a chemical element of the periodic table, located in the group 12 and the period 4, and is having the atomic number 30.
The atomic number of an element is equal to the number of protons and electrons in that element. The positive valent is the electron that is attracted to the positive charge of the zinc atom. Chemical element, zinc, information from authoritative sources.









