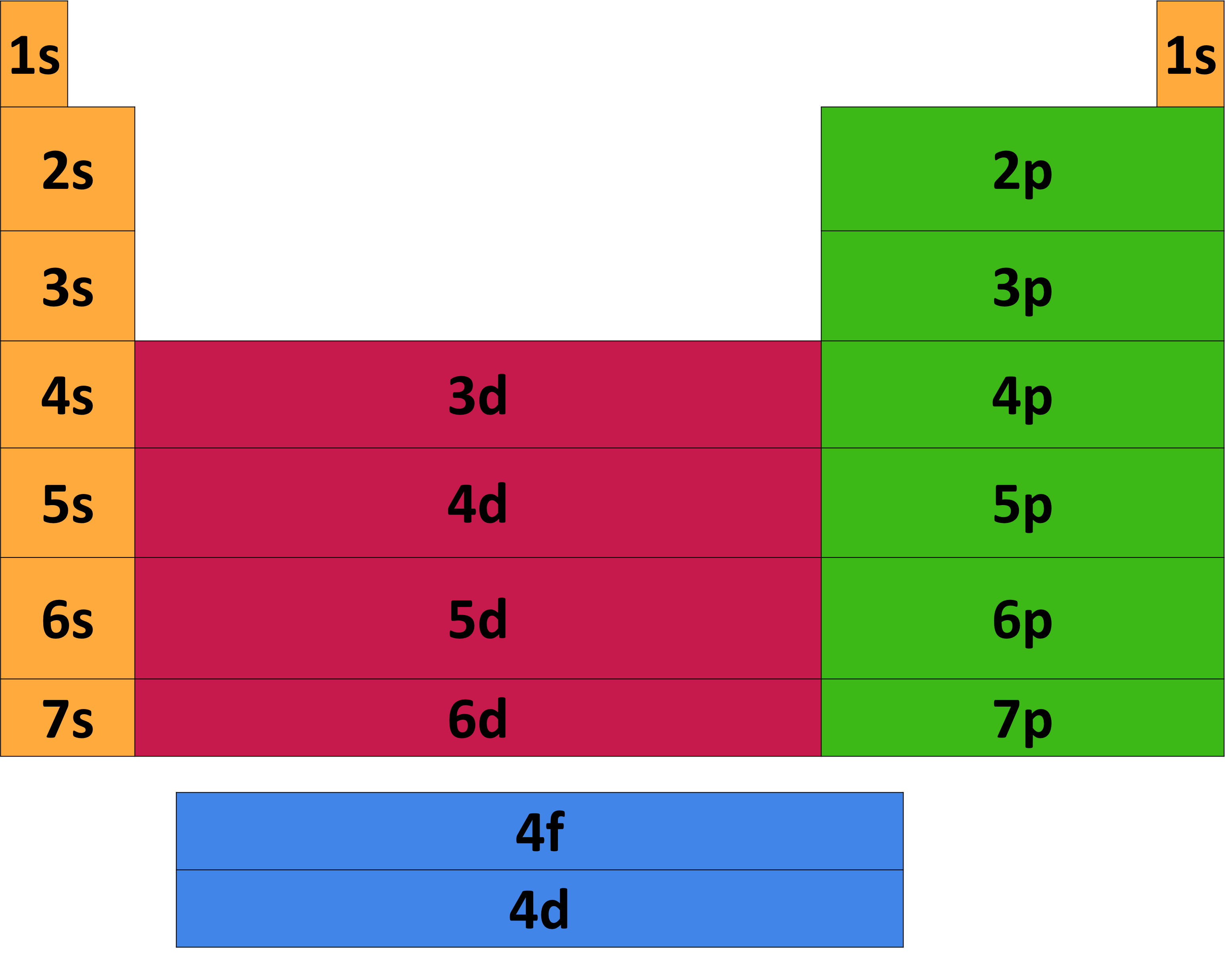
Elements in the second row of the periodic table place their electrons in the 2n shell as well as the 1n shell.
Periodic table and energy levels. Students match seven categories of energy levels in a blank periodic table of elements in this cutout. The energy level periodic table is a collection of formulas that help people understand how energy moves about the earth. The highest energy level number (1 through 7) for the electrons in an atom corresponds to the period (or row) in the.
The periodic table is an attempt to explain the relationship between different atoms and how they relate to one another. The 5g 6f and 7d orbitals should have about the same. After plugging in the values of constants, the equation becomes:
Two fill the orbital, and the third is placed in. Ionization energy of carbon (c) 11.26 ev. The names of the periods in periodic table are;
For instance, lithium () has three electrons: Click on any element's name for further information on chemical. N is the principal quantum number (or energy level) of the electron;
After the third energy level has 8 electrons (argon), the next 2 electrons go into the fourth energy level. What is the highest energy level on the periodic table? Web the seven rows of the table are called a periodic table.
The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements. The 2s has lower energy when compared to 2p. 3.2 inorganic chemistry periodicity the periodic table provides chemists with a.









