All of the methods give the same answer, though, so you can choose whichever approach you prefer!
Determining limiting reactant. Calculate the number of moles h 2 = given volume /molar volume. However, if the amount of. There are two ways to determine the limiting reagent.
Determining limiting reactants students use a pressure sensor to experimentally determine the limiting and excess reactant when the amount of one reactant is varied and analyze data to. 4hf (g) (this signifies that 4 moles of hf are required to completely consume 1 mole of sio2). Click create assignment to assign this modality to your lms.
Determine the limiting reagent and amount of excess reagent present if the mass of na = 23 and cl = 35.5. Determining limiting reactants students use a pressure sensor to experimentally determine the limiting and excess reactant when the amount of one reactant is varied and analyze data to. There are two ways of determining which reactant is the limiting reactant:
By comparing the ratio of reactants to each other next to a balanced chemical equation or by. As the given reaction is not balanced, so its balanced form is as follows:. N2 + h2 → nh3 solution:
How to find limiting reagent and excess reactant for the limiting reactant equation given below: Compare the mole ratio of the reactants with the ratio in the balanced chemical equation to determine which reactant is limiting. In order to find the limiting reactant in a chemical equation i usually convert grams to moles and then use the molar ratio based on their coefficients to compare the two.
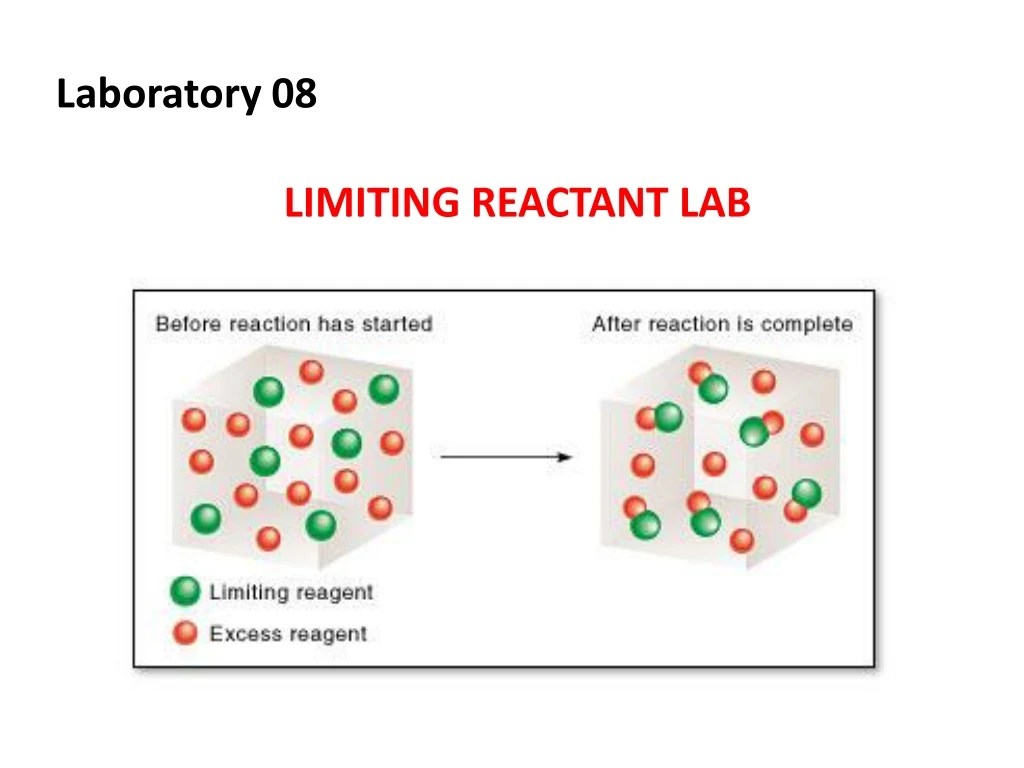
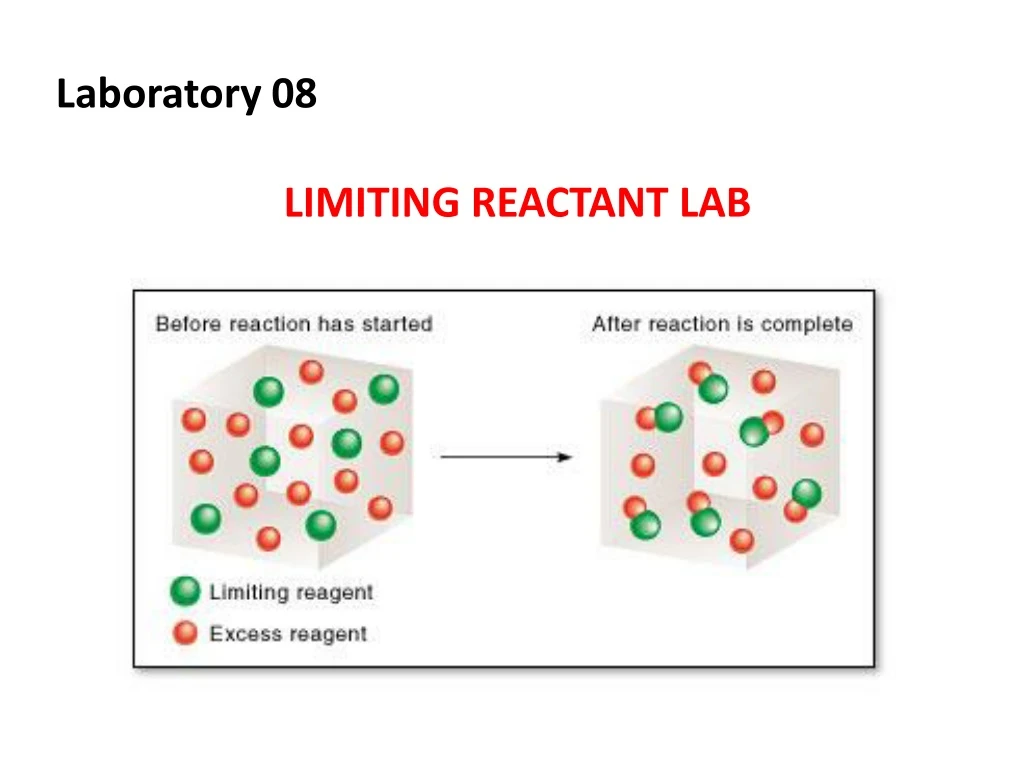
With that, the mole numbers of. Limiting reactant is a chemical reactant which limits the quantity of formed product. Determine the number of moles of each reactant.