Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and.
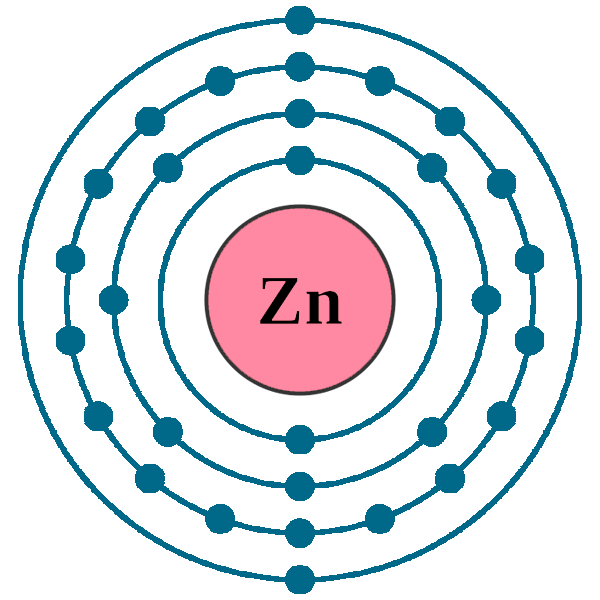
Write the complete electron configuration for the zinc atom. Using noble gas notation write the electron configuration for the zinc atom. Full electron configuration electron shell arrangement; Write the complete electron configuration for the zinc atom.
Therefore, the number of protons is equal to the number of electrons. Write the complete electron configuration for the zinc atom. Electron configuration of helium (he) 1s 2:
Copper ← zinc → gallium. The atom is electrically neutral. The electronic configuration of zinc is 1s2,2s2,2p6,3s2,3p6,4s2,3d10.
The electronic configuration of each element is decided by the aufbau principle which states that the electrons fill orbitals in order of increasing energy levels. Its electrons are filled in the following order: Using noble gas notation write the electron configuration for the calcium atom.
1s2 2s2 2p6 3s2 3p6 3d10 4s2. Write the complete electron configuration for the zinc atom. Kindly login to access the content at no cost.
The atomic number of oxygen is 8, implying that an oxygen atom holds 8 electrons. Write the complete electron configuration for the zinc atom using noble gas notation write the electron configuration for the chromium. 1.)the ground state electronic configuration of calcium (z=20) is 1s22s22p63s23p64s2.









