It is stable in the presence of oxygen, but it is unstable in low oxygen conditions.
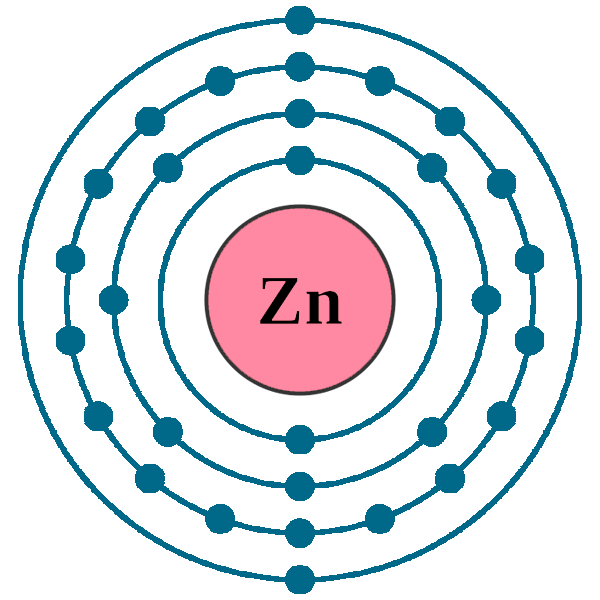
Which is the electron configuration for zinc. 1 answer anor277 may 24, 2016. Zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. What is the outer electron configuration for zinc?
Chemistry electron configuration electron configuration. Zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure.the chemical symbol for zinc is zn. Located in the iv period.
5 rows the zinc atom donates two electrons in the 4s orbital to form a zinc ion (zn 2+ ). The chemical symbol for zinc is zn. Ground state electron configuration of zinc (zn):.
Electronic configuration of the zinc atom in. Copper ← zinc → gallium. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the.
Zinc alloys have been used for centuries;. The electronic configuration of an element is a symbolic notation of the manner in which the electrons of its atoms are distributed over different atomic orbitals. What is zinc's electron configuration?
The atomic number of zinc is 30, so 30 electrons are present in its neutral state, so configuration in neutral. In this case, the zinc atom carries a. It is a moderately reactive metal and strong reducing agent.








