It is important to remember that solving stoichiometry problems is very similar to following a recipe.
What is the first step in most stoichiometry problems. C 6 h 12 o 6 → 2c 2 h 5 oh + 2co 2. Answeryou have to do stoichiometry. Explain how to solve each type of stoichiometry problems.
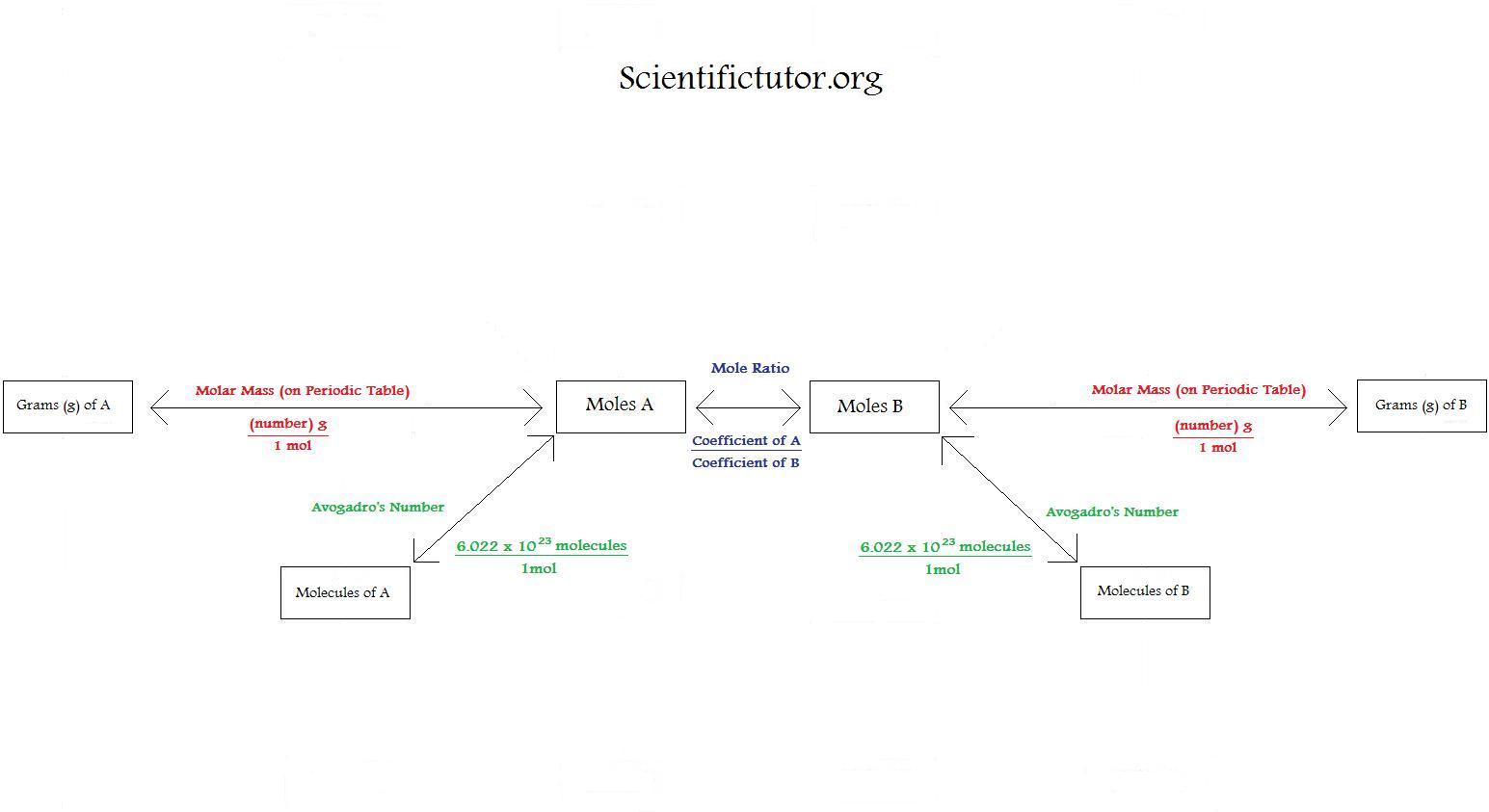
The mole allows a chemist to find what masses of substances to use in a reaction. If 2.5 moles of c 6 h 12 o 6 reacts, how many moles of carbon. Convert the given mass or number of.
1 mole h= 6.02*10^23 atoms h. The first step in most stoichiometry problems is to balance the chemical equation. The first step in any stoichiometric problem is to always ensure that the chemical reaction you are dealing with is balanced, clarity of the concept of a ‘mole’ and the relationship between ‘amount.
At the center of stoichiometry is the mole. 1 mole c6h10oh6= 10 mol hsecond step: In this video, we will look at the steps to solving stoichiometry problems.
Mathematical relationship between the number of reactants and products. The reactant that determines the amount of product that can be formed in a reaction. Using the mole ratio, calculate the.
Convert units of a given substance to moles. One mole is an amount of a substance that contains 6.022 ×.









