Recognize characteristics of monatomic and polyatomic ions.
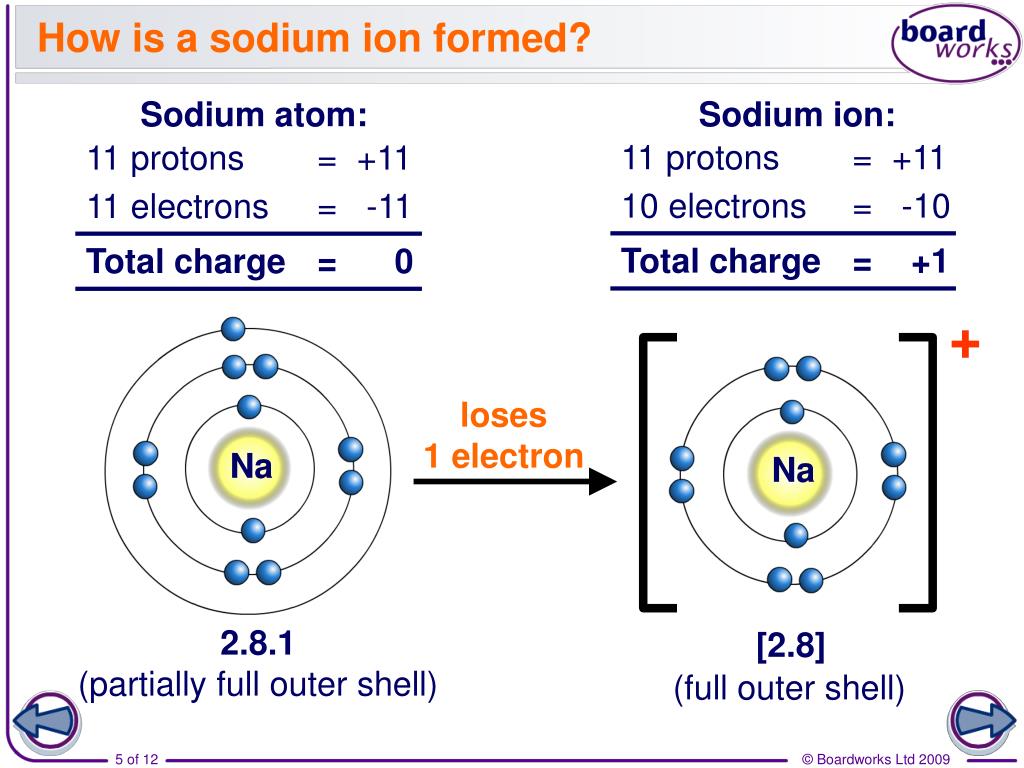
What is the charge formed on neon ions. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. But since it can be ionized, this is the energy required to give a mole of neon a. Web 93 rows this electric charge generated on the ion is known as ionic charge.
Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble gases. Web learning outcomes define ion, cation, and anion. Web by the same token, chlorine will be isoelectronic with argon if it gains one electron, but will have to lose seven electrons to be isoelectronic with neon.
Web define ionic compounds predict the type of compound formed from elements based on their location within the periodic table determine formulas for simple ionic. By combination of ions with other. Electron transfer between lithium (li) and fluorine (f).
Web when the inner transition metals form ions, they usually have a 3+ charge, resulting from the loss of their outermost s electrons and a d or f electron. Web helium, neon, argon, krypton and xenon are in group 0. When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet.
Web the energy released when an electron is added to the neutral atom and a negative ion is formed. They all have a full outer electron shell which means they are already stable and so do not need to lose or gain electrons. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the.
As mentioned above, there are positive and negative ions. Forming an ionic bond, li and f become li + and f − ions. Electronegativity (pauling scale) the tendency of an atom to attract electrons.







