These circular paths are called orbit (shell).
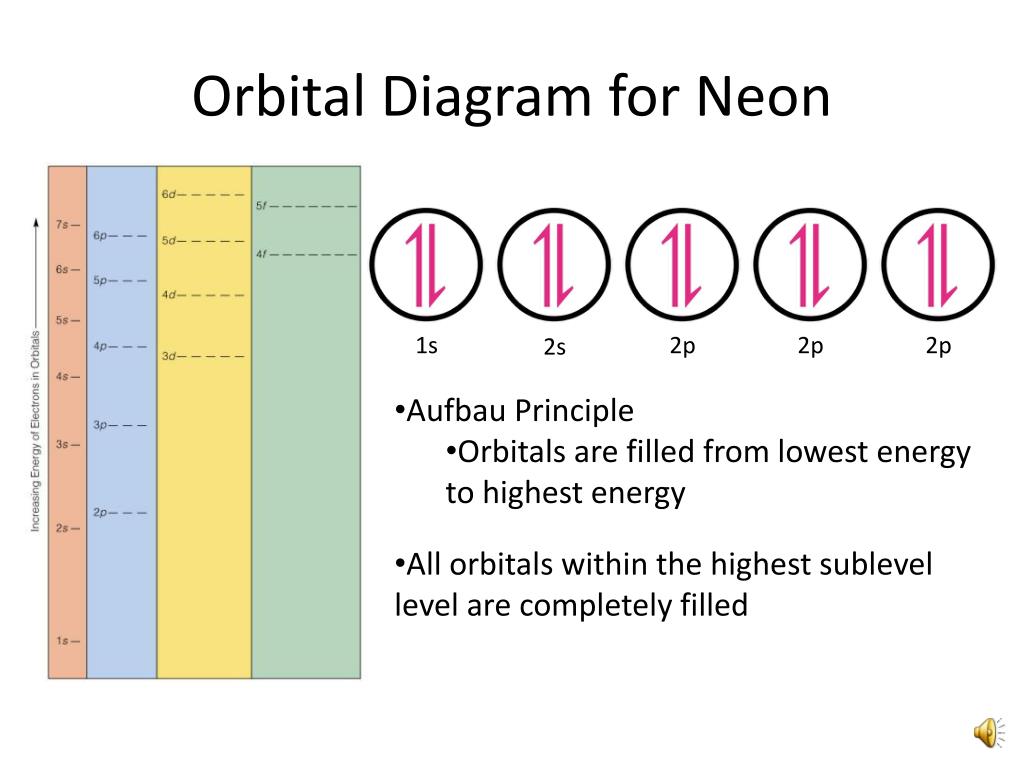
What is neon orbital notation. Neon is the tenth element with a total of 10 electrons. The electron configuration of neon is 1s 2 2s 2. Web orbital notation is a way of writing an electron configuration to provide more specific information about the electrons in an atom of an element.
Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. Read an integer from the user or initialize a number ( n) to check. Web for example, sodium has one 3s electron in excess of the noble gas neon (chemical symbol ne, atomic number 10), and so its shorthand notation is [ne]3s 1.
An orbital notation is a specific way to show the arrangement of electrons in an atom using what are called subshells. Web commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation. Elements are organised into blocks by the orbital type in which the outer electrons are.
Web for example, the electron configuration of the neon atom is 1s2 2s2 2p6, meaning that the 1s, 2s and 2p subshells are occupied by 2, 2 and 6 electrons respectively. Web the arrangement of electrons in neon in specific rules in different orbits and orbitals is called the electron configuration of neon. Web neon is a chemical element with the symbol ne and atomic number 10.
Similarly, fluorine has the electron configuration 1s 2 2s 2 2p 5: It is a noble gas. Web so, a neon atom has.
Web neon is the tenth element with a total of 10 electrons. Web because all the 2p orbitals are degenerate, it doesn’t matter which one has the pair of electrons. [11] neon is a colorless, odorless, inert monatomic gas under standard conditions, with.







