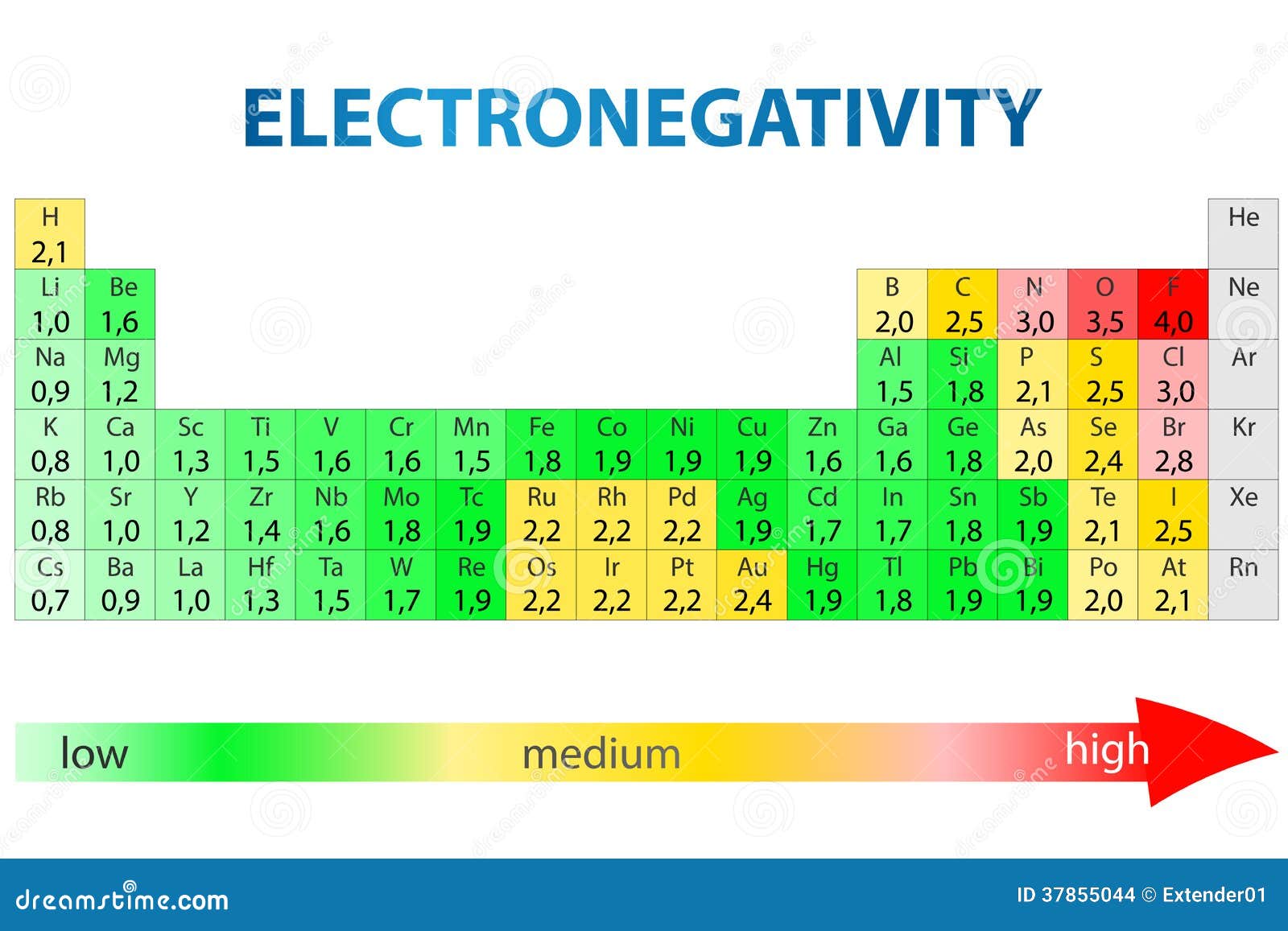
Web electronegativity is a kind of measure that tells how strongly atoms attract the bonding electrons to them.
What is neon electronegativity. Web 26.11.2021 by author electron affinity and electronegativity of neon electron affinity of neon is — kj/mol. The ions [near] , [neh] , and [hene] have been observed from optical and mass spectrometric studies. Thus, fluorine is the most electronegative element, while francium is.
Web neon is a chemical element with atomic number 10 which means there are 10 protons and 10 electrons in the atomic structure. First ionization energy of neon is. Web electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons.
Web electronegativity is a measure of an atom’s attraction for the electrons in a bond. The chemical symbol for neon is ne. In general, the elements in the upper right.
Web electronegativity is an important chemical property that tells how an atom of neon may atract electrons from other atoms and form a bond. Web 103 rows electronegativity 1: Electronegativity of neon is —.
As is the case with its lighter analogue, helium, no strongly bound neutral molecules containing neon have been identified. Values for electronegativity run from 0 to 4. Electronegativity is used to predict whether a bond between atoms will be ionic or.
Web 119 rows electronegativity is a chemical property which describes how well an atom can attract an electron to itself. The electronegativity of an element refers to the ability of the given element to attract shared electrons in a bond. Across a period from left to right the electronegativity of atoms increases.


.PNG)




