The best way to study the ions of any element is to study its electron configuration process.
What is neon electron configuration. Electron configuration chart of all elements is mentioned in the table below. As is the case with its lighter analogue, helium , no strongly bound neutral molecules containing neon have been identified. [he]2s2 2p6 ●longhand electron configuration for neon:
1s 2 2s 2 2p 6. Web 【】what is the neon (ne) electron configuration? Electron configuration is basically a distribution of electrons for the atom in the molecular or the atomic orbital.
Possible oxidation states are 0. 1s2 2s2 2p6 ●neon electron configuration long form :1s2 2s2 2p6 ●neon electron configuration. Web what is the electronic configuration of neon?
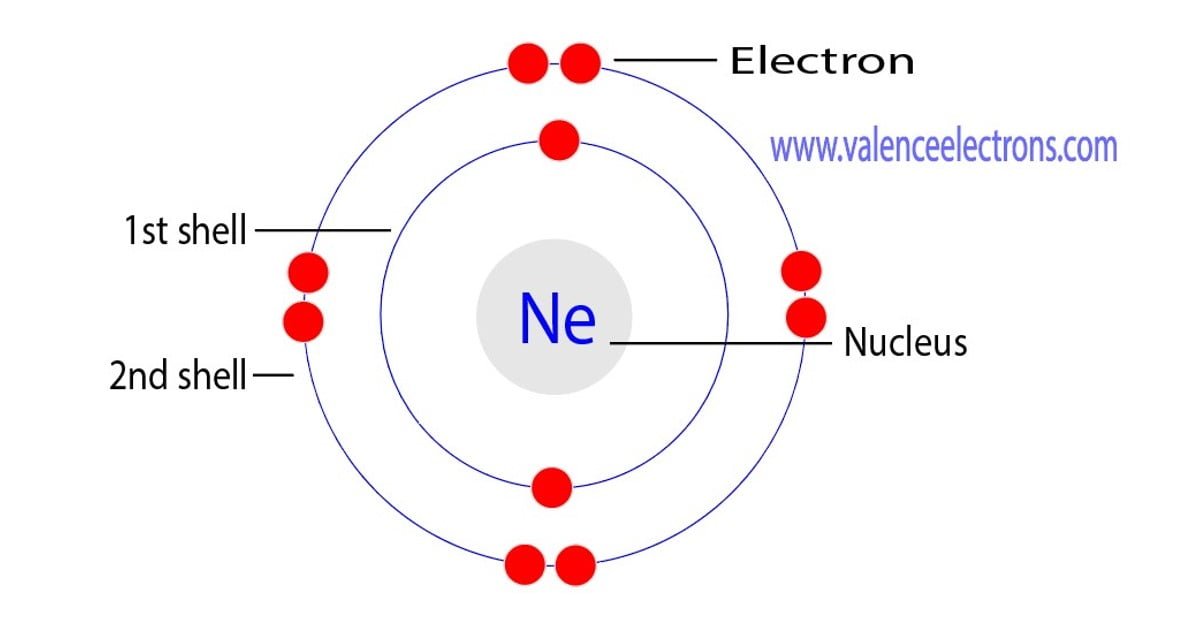
Neon is gaseous in nature and exhibits an orangish red glow in an electric field. Web the atomic number of neon represents the total number of electrons of neon. Web neon electron configuration:
Well, in chemistry the ions are basically those groups of atoms that may either gain or lose electrons. Web neon, chemical element, inert gas of group 18 (noble gases) of the periodic table, used in electric signs and fluorescent lamps. So, the electron configuration of neon is 1 s 2 2 s 2 2 p 6 now, the neon atom have fully filled valence shell.
Neon has atomic number 10, so a neon atom has 10 protons in its nucleus and therefore 10 electrons. Subsequently, these atoms groups may have both positive and negative charges upon them. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25):
![[5 Steps] Electron Configuration for or of Neon in Just 5 Steps](https://i2.wp.com/2.bp.blogspot.com/-jMs9FCVMeSw/XD4FtZiSVlI/AAAAAAAAYZg/rJnlLKM6S6EHv8FmJZr4pj2PmzTZjk0hQCLcBGAs/s1600/20190115_220744.jpg)






