When an electron is placed in the lowest stationary state/ energy level possible, it is said to be in the ground state.
What is energy level in periodic table. The first chemical element is cesium and the last one is helium. What are energy levels on the periodic table. Electrons in this energy level revolve in the orbit having the smallest possible.
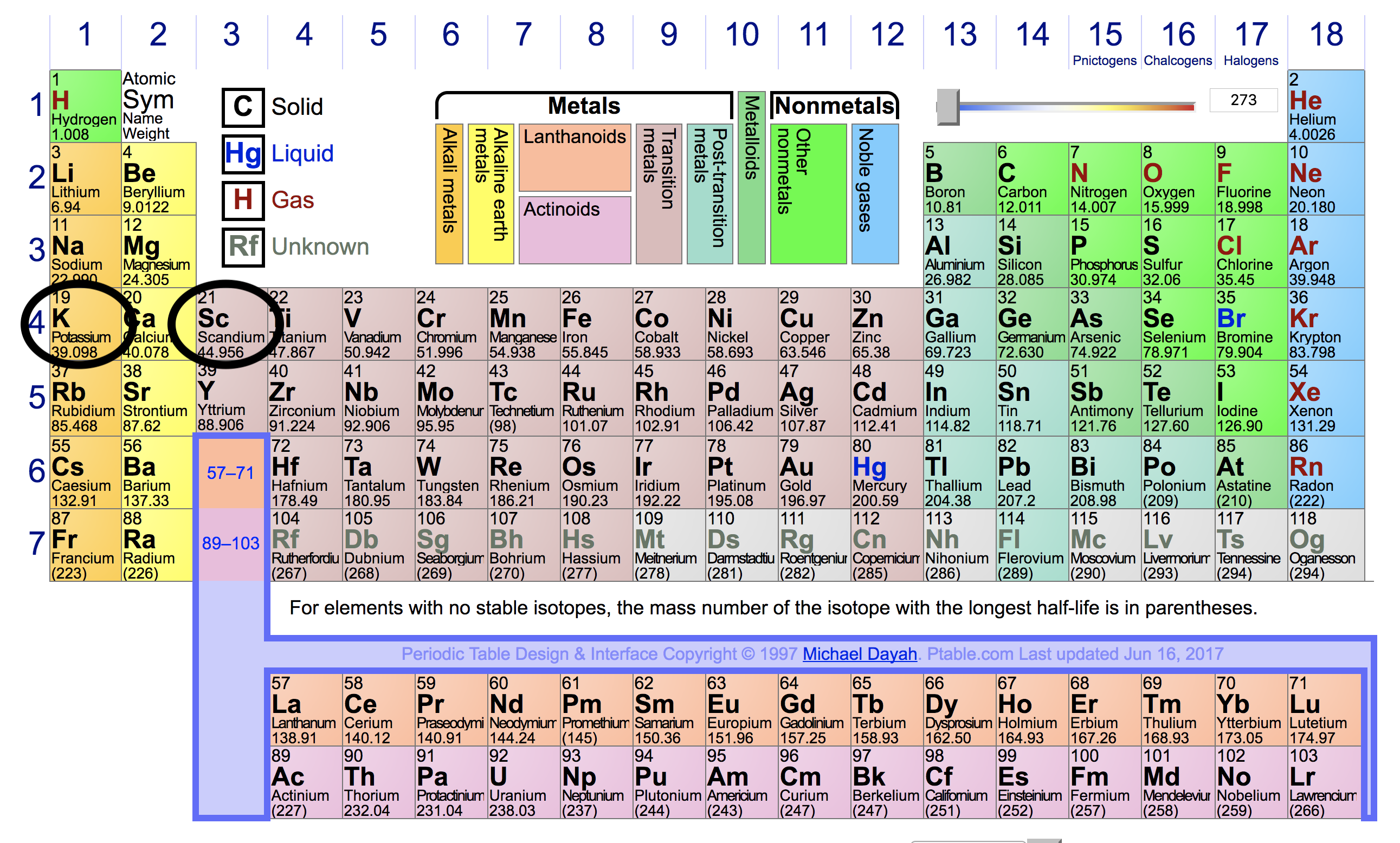
The tabular chart on the right is arranged by ionization energy. The highest energy level number (1 through 7) for the electrons in an atom corresponds to the period (or row) in the periodic table to which that atom belongs. Web the seven rows of the table are called a periodic table.
The highest energy level number (1 through 7) for the electrons in an atom corresponds to the period (or row) in the. Because there are 7 periods in. In chemistry, the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atom's nucleus.
The 5g 6f and 7d orbitals should have about the same. What is the highest energy level on the periodic table? The first period of the periodic table's elements all have 1 energy level.
The energy level periodic table is a collection of formulas that help people understand how energy moves about the earth. For example, energy level i can hold a maximum of two electrons, and energy. Wavefunctions of a hydrogen atom, showing.
You are wondering about the question what are energy levels on the periodic table but currently there is no answer, so let kienthuctudonghoa.com summarize and list the top articles with the. Ionization energy is the amount of energy necessary to remove an electron from an atom. 119 rows for chemistry students and teachers:









