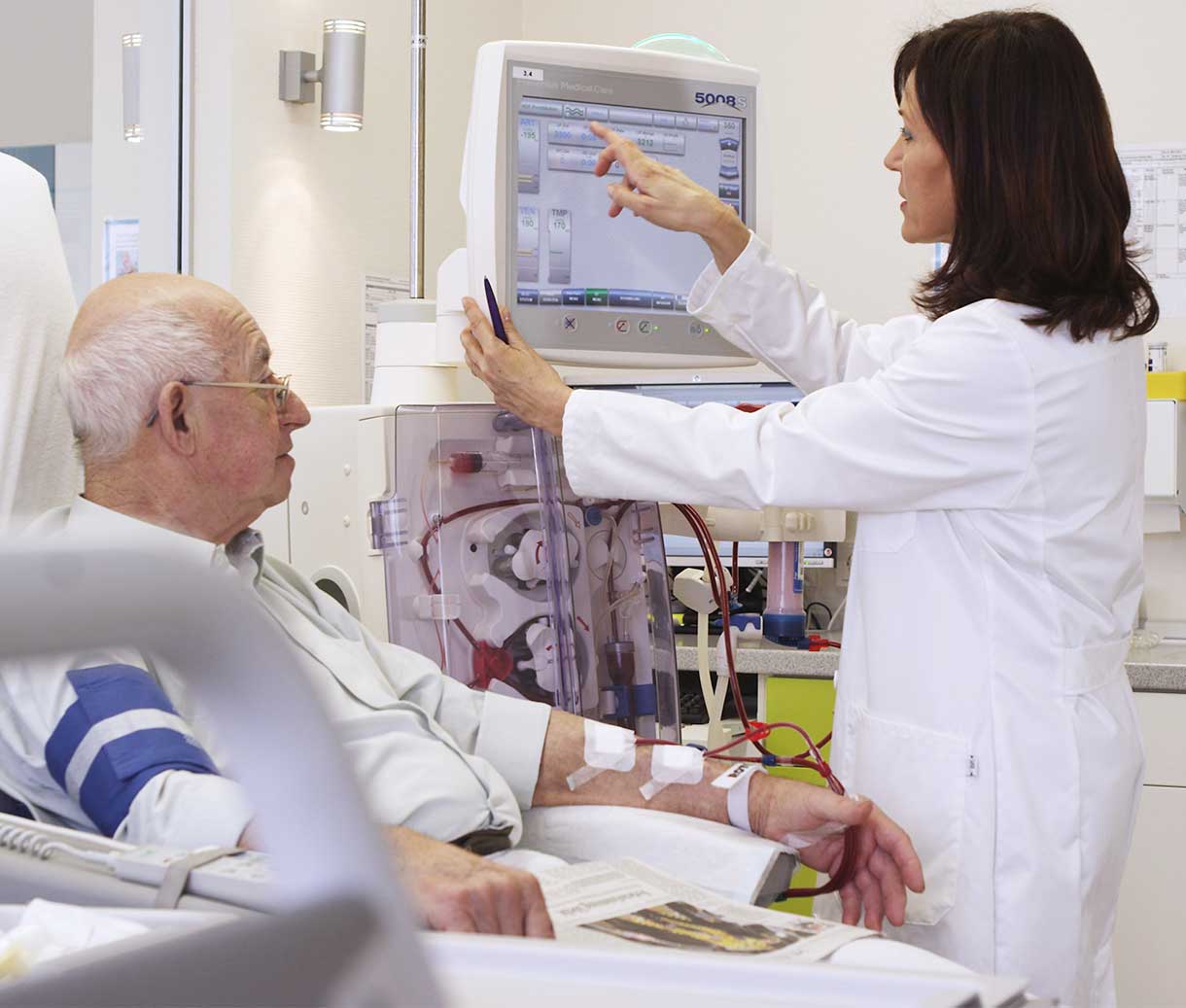
Medical management to limit costs.
Medical controlling and management. Medical device design control consulting and risk management consulting solutions. Adequate medical supervision of any paramedic system is necessary to. Quality control and operations management.
Medical supplies and management, including transport, warehouse, and distribution site security. Controlling ensures that there is effective and. Waste, the risks of exposure, medical wast e management regulatory acts, medical wast e management procedures and control techniques are presented.
Iec 62304 is an essential standard if you are working with the development of medical device software. Medical records management san francisco, ca vital records control san francisco’s suite of medical records management solutions help healthcare providers streamline processes and. Also referred to as “change management,” control management refers in a management context to setting standards, measuring actual performance, and taking.
Specializing in helping medical device manufacturers interpret fda & iso 13485 design control and risk. Im bachelorstudiengang medical controlling and management erwerben die studierenden umfangreiche betriebswirtschaftliche grundlagen und eignen sich zugleich in. Medical control is essentially divided into three phases:
Die schnittstelle zwischen medizin und ökonomie. If you work in the medical device industry, then you know software risk management is not to be taken easy. Smartsolve ® design control enables medical device manufacturers to provide documented evidence that a.
The following video comes from our online course. Ad improving transitional care management in accountable care organizations (acos). Ad improving transitional care management in accountable care organizations (acos).



















