Draw the electron configuration for a neutral atom of zinc.
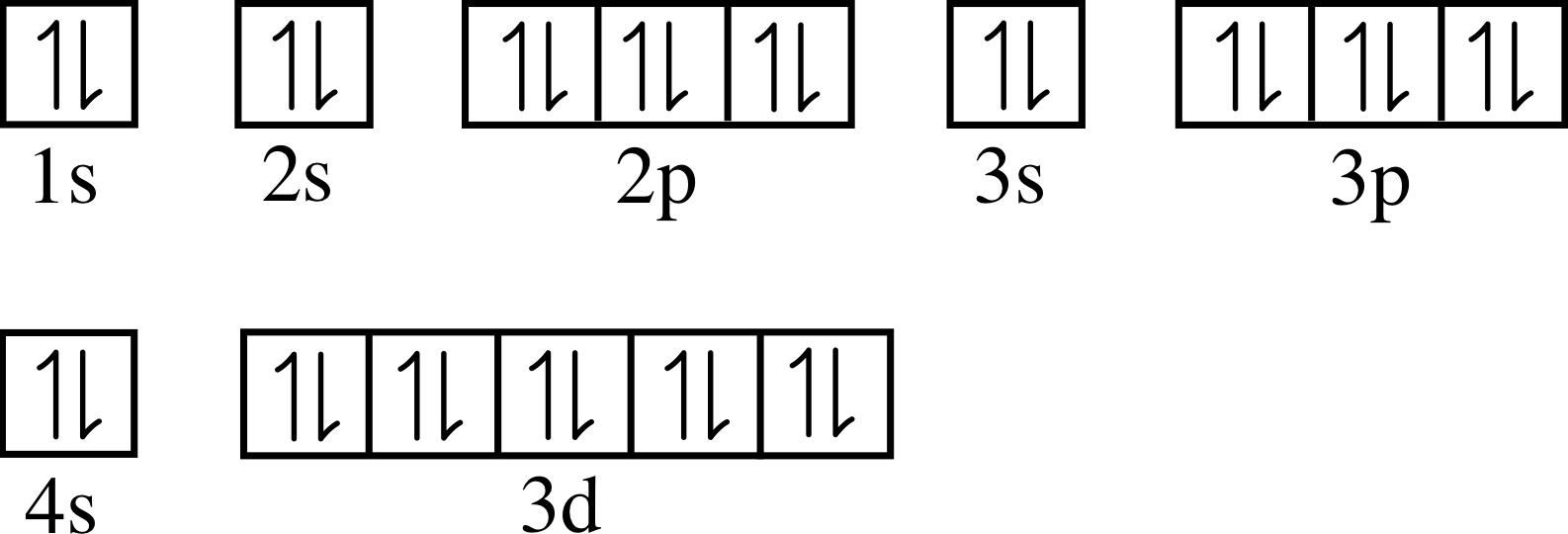
Ground state electron configuration zinc. The ground state electron configuration of zinc is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2. Electronic configuration of zinc zn:. The zinc atom has 30 protons ⇒ 30 electrons.
The ground state electron configuration of ground state gaseous neutral zinc is [. For example, using carbon as the example. Zinc ion (zn 2+) electron configuration.
We first need to find the nu. 1 s 2, 2 s 2, 2 p 6, 3 s 2, 3 p 6, 4 s 2, 3 d 1 0. Ground state electron configuration of zinc (zn):
1s 2 for writing ground state electron configurations, a few main steps should be followed. The aufbau principle states that, in a ground state electron configuration, the lowest energy orbitals are filled with electrons first. The electron configuration for zinc is 1s2 2s2 2p6 3s2 3p6 4s2 3d10.
Zinc atoms have 30 electrons and the shell structure is 2.8.18.2. The ground state electron configuration of ground state gaseous neutral zinc is [ar]. 1s2 2s2 2p6 3s2 3p6 3d10 4s2.
What is electron configuration for zinc? Transition metals with an oxidation state. Find the amount of electrons in the atom.







