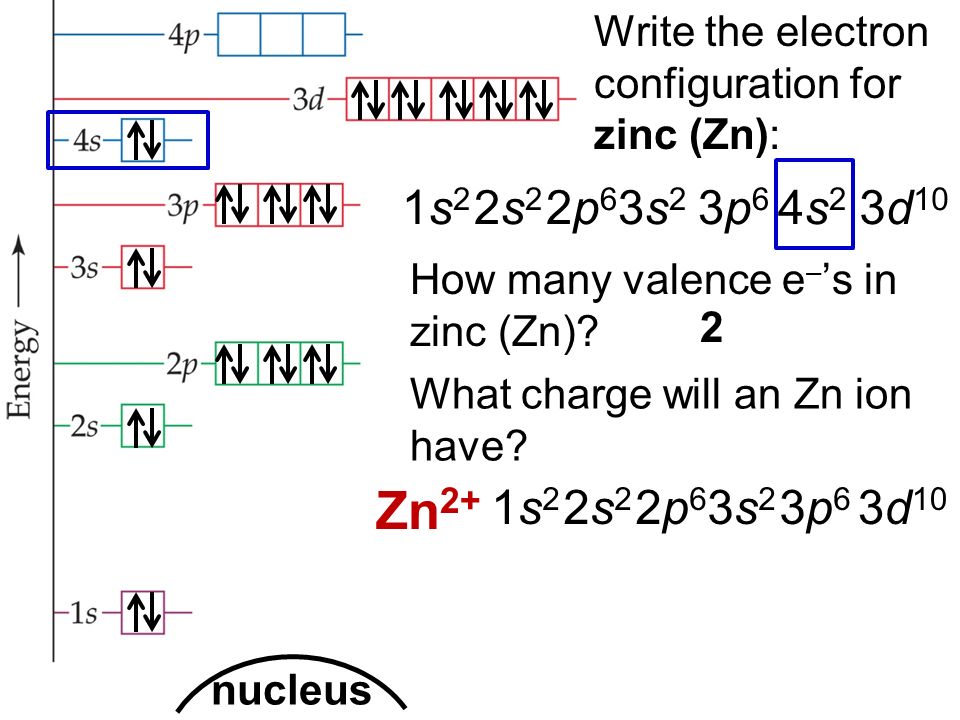
The correct electron configuration of zn 2+ is 1s2 2s2 2p6 3s2 3p6 3d10 or.
Ground state electron configuration for zn. The zinc atom donates two electrons in the 4s orbital to form a zinc ion (zn 2+ ). The atomic number of zinc is 30. 1s 2 for writing ground state electron configurations, a few main steps should be followed.
Also what is the ground state electron configuration of zn? The zn belongs to period 4 of the periodic table.it has the atomic number 30. (a)[ar]4s13d 10 (b) [ar]3db (c) [ar]4s23d (d) [kr]4s23f1 (e) none of these 16.
Thus, the maximum number of electrons in the ground state of configuration of zn which have l = any positive integral value and m = any non zero value. Total = 4 + 4 + 8 = 1 6 electrons. We first need to find the nu.
The given element is zinc. The ground state is where electrons are in the lowest possible energy while the excited state is where the electron is excited and jumps into a higher orbital. To write the configuration for the zinc and the zinc ion, first we need to write the electron configuration for just zinc (zn).
Its abbreviated electron configuration is [ar]4s23d10 the full electron configuration is 1s22s22p63s23p64s23d10 (configurations for. Therefore, the number of protons is equal to the number of electrons. 4s2, with 1s0 as the.
Which of the following elements is chemically. Ge, fe, zn, ni, w, tl. Here, the electron configuration of zinc ion (zn 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10.









