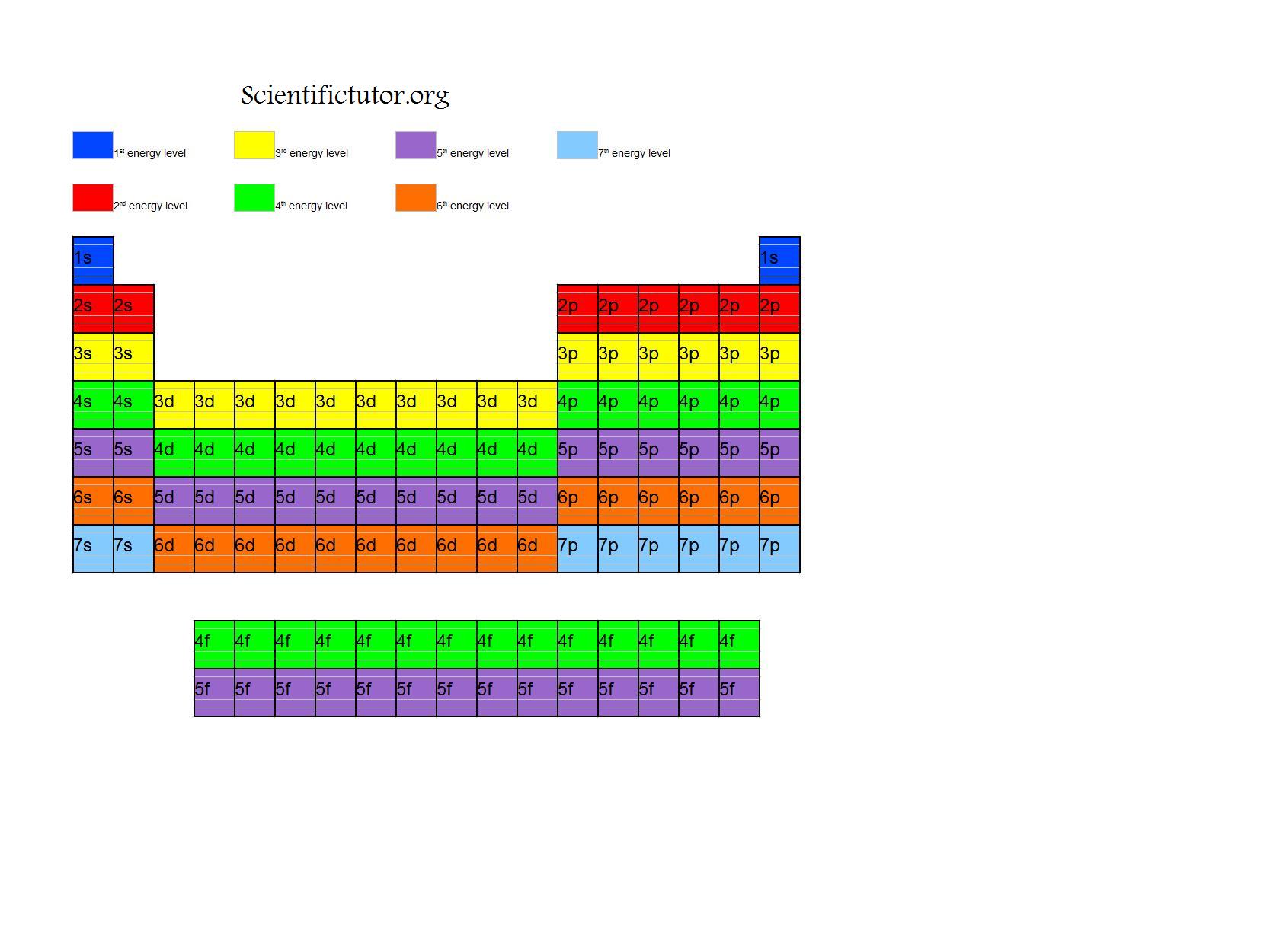
Energy level diagram is a part of cbse class 11 chemistry first term syllabus.
Energy level of an element. At energy level 2, there are both s and p orbitals. The energy levels are arranged according to the nearer to the nucleus ( from inside to outside), ( k, l, m, n, o, p, q), each level has a certain amount of energy that increases by. According to bohr's theory, electrons of an.
Each electron will exist in a ground state energy level, n=0, until it is excited, or. The 2s has lower energy when compared to 2p. In order to fill the electrons in various atomic orbitals, we need to know how the energy levels vary as the nuclear charge increases.
The nucleus of a hydrogen atom has a charge of +1. An energy level in an atom is defined as the energy an electron has that is above ground state. R(n) = n 2 x r(1) an atomic.
The energy of the electron in a hydrogen atom depends only on the principal quantum number, n. Two energy levels, 16t 2g and 13e g, existing around 2.5 ev,. It carries a total of 12 periods and 5 to 6 marks.
When you look at the periodic table of the elements, the energy levels of the atoms correspond to the rows of the table. The energy of a 250nm radiation is. How to find work function of an element when threshold energy, wavelength is given?
In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. This photon energy must be the difference between two energy levels for a hydrogen electron, since that is the amount of energy released by the electron moving from one level to. Each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the particular element.









