Both elements exhibit only one normal oxidation state (+2), and the zn2+ and.
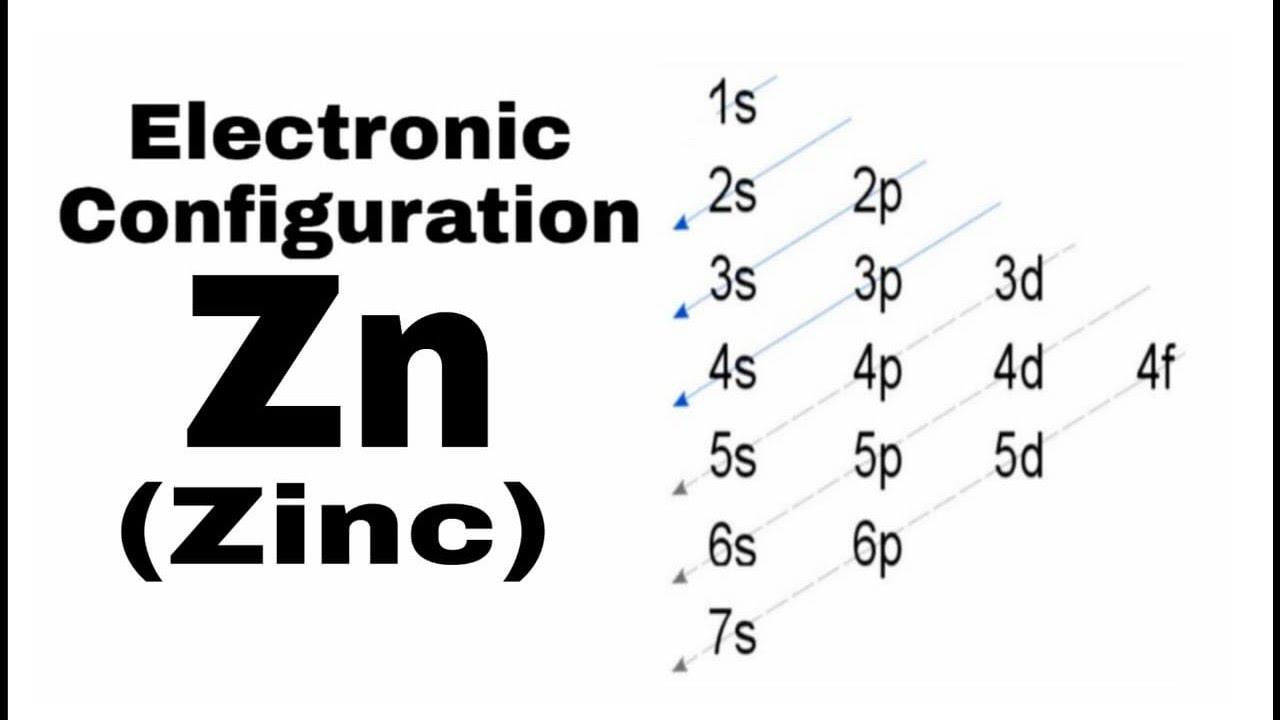
Electron configuration zn. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. The electron configuration states where electrons are likely to be in an atom. 5 rows the zinc atom donates two electrons in the 4s orbital to form a zinc ion (zn 2+ ).
The electron configuration for zinc is 1s2 2s2 2p6 3s2 3p6 4s2 3d10. Electron configuration was first conceived under the bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the. The electronic configuration of each element is decided by the aufbau principle which states that the electrons fill orbitals in order of increasing energy levels.
Here, the electron configuration of zinc ion is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10. 1 answer anor277 nov 22, 2017 by taking the atomic. The chemical symbol for zinc is zn.
What is the ground state electron configuration for zinc? Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and. Five stable isotopes of zinc occur in nature, with 64 zn being the most abundant isotope (49.17% natural abundance).
In some respects zinc is chemically similar to magnesium: The full electron configuration of zinc is 1s2 2s22p6 3s23p63d10 4s2zinc, also written zinc, is defined as the chemical element that belongs to the periodic table of elements. The chemical symbol of zinc is 'zn'.
Copper ← zinc → gallium. It is a moderately reactive metal and strong reducing agent. Ground state electron configuration of zinc (zn):.









