The electronic configuration of the zinc ion is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10
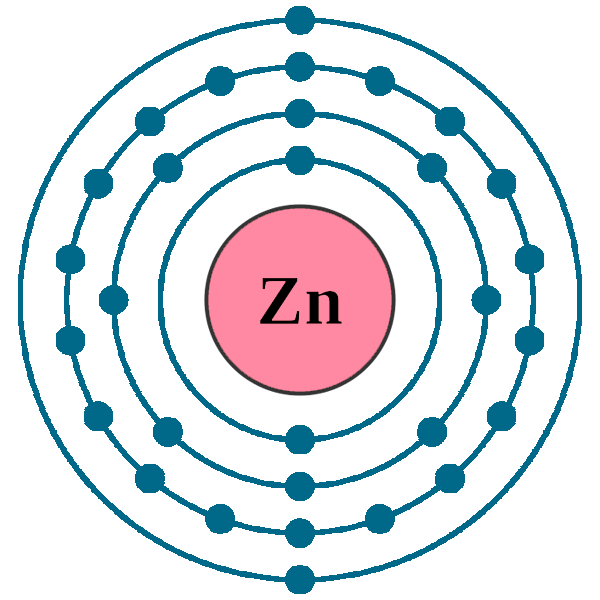
Electron configuration of zinc ion. The electronic configuration of zinc is 1s2,2s2,2p6,3s2,3p6,4s2,3d10. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and. Zn (zinc) is an element with position number 30 in the periodic table.
Electronic configuration of the zinc atom in. The zinc atom donates two electrons in the 4s orbital to form a zinc ion (zn 2+ ). Electronic configuration of zinc ion zn 2 +:
When compounds in this oxidation state are formed, the outer shell s electrons are lost, yielding a bare zinc ion with the. By the time the 3 d orbitals are filled with electrons, the 4 s orbitals have become higher in energy, making it easier to remove them. All electrons are paired, and therefore zn (0) is diamagnetic.
Zn loses its two electrons and results in the formation of zn 2 + ion. Copper ← zinc → gallium. The same rule will apply to transition metals when forming ions.
1s2 2s2 2p6 3s2 3p6 3d10 4s2. Electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas configuration) as well as full. The zinc atom donates two electrons in the 4s orbital to form a zinc ion(zn 2+).
Selenium ion electron configuration is. Here, the electron configuration of zinc ion (zn 2+) is 1s 2 2s 2 2p 6 3s 2. Zn can only form stable cation, zn2+ by releasing two electrons from its outermost shell.









