When solid kbr is dissolved in water, the solution gets colder b.
Are the following processes exothermic or endothermic. The other two processes (iii) and (iv) are of. Determine if the net flow of energy (heat) is into or out of. This question has multiple correct options.
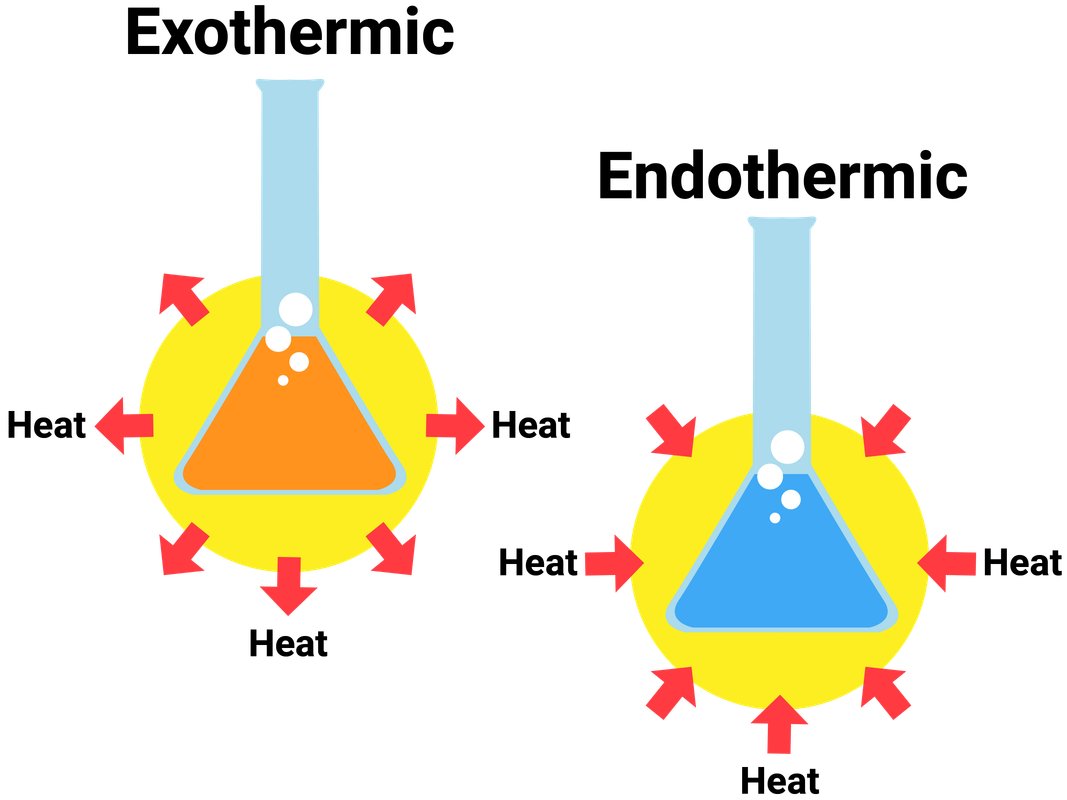
Endothermic reactions need an input of energy (in the form of heat) for the reaction to start. Phase transitions occur when energy is gained or released by a material, resulting in either more or less order arrangements of molecules. Test prep · mcat · foundation 5:
Which among the following processes are exothermic reactions? In general, the focus in this chapter is going to be on. The input of heat energy, from the surroundings, triggers the breakdown of chemical and physical.
(a) solid kbr dissolves in water and the solutions gets colder (b) natural gas is burned in a furnace. Which of the following processes is endothermic? Thus in an exothermic reaction, energy is transferred into the surroundings.
Combustion is always exothermic, releasing heat.b. Evaporation of alcohol b this quiz will give you understanding of the basic properties and differences of exothermic and endothermic. Are the following processes exothermic or endothermic?
(a) combustion of methane (b) melting of ice (c) raising the temperature of water. When solid kbr is dissolved in water, the solution gets colder. The terms endothermic and exothermic are used to describe the heat transfer during different processes including chemical reactions.
/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png)








