The electron configuration shows the distribution of electrons into subshells.
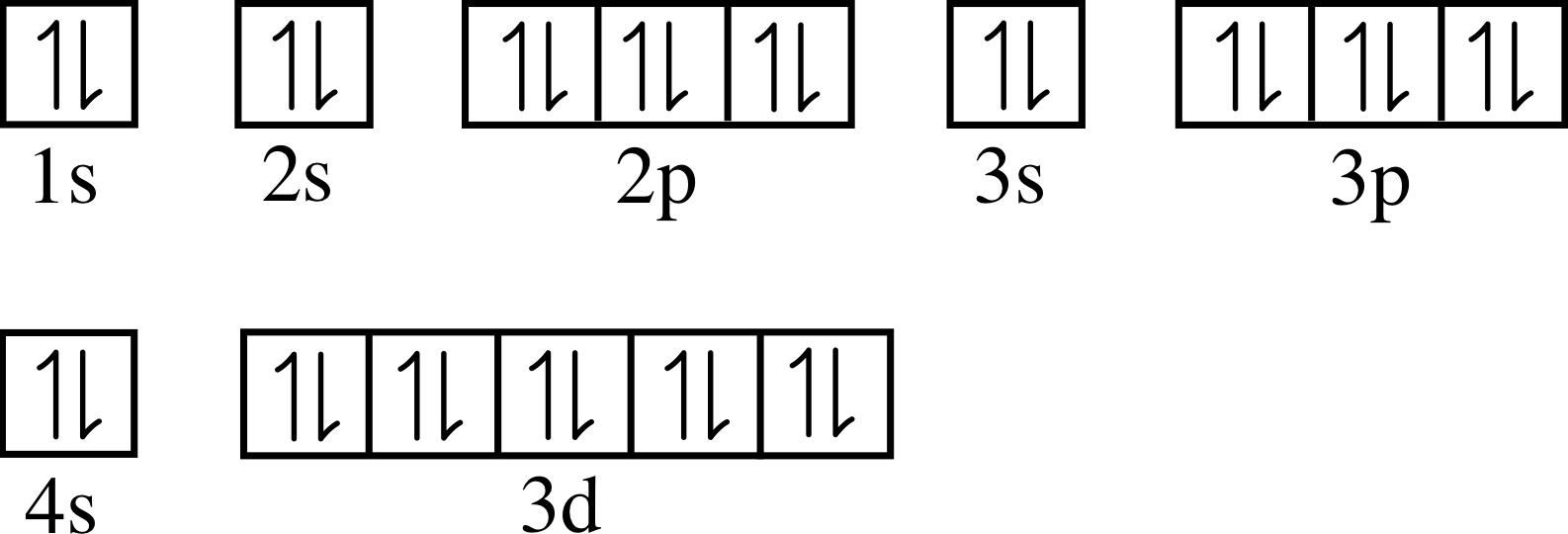
Zinc full electron configuration. There are 30 electrons in an atom of zinc because the atomic number of zinc is 30. 1s2 2s2 2p6 3s2 3p6 3d10 4s2. Copper ← zinc → gallium.
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 >> back to key information about the elementback to key information about the element The full electron configuration of zinc is 1s2 2s22p6 3s23p63d10 4s2zinc, also written zinc, is defined as the chemical element that belongs to the periodic table of elements. Chemistry electron configuration electron configuration.
5 rows the zinc atom donates two electrons in the 4s orbital to form a zinc ion (zn 2+ ). The zinc atom donates two electrons in the 4s orbital to form a zinc ion (zn 2+ ). Here, the electron configuration of zinc ion (zn 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6.
This list of electron configurations of elements contains all the elements in increasing order of. Zn (zinc) is an element with position number 30 in the periodic table. Full electron configuration of zinc:
The electron configuration of zn(0), or zinc metal, is [ar] 3d10 4s2. Also in crookesite, hutchinsonite and lorandite. Electronic configuration of the zinc atom in.
Electronegativity of zinc is 1.65. The zinc atom donates two electrons in the 4s orbital to form a zinc ion(zn 2+). It is a moderately reactive metal and strong reducing agent.









![Electron orbitals [20101215] Electrons, Awkward, Rearrange](https://i2.wp.com/i.pinimg.com/originals/89/ab/8f/89ab8fae9b13b38e354593c867394935.png)