Ground state electron configuration of zinc (zn):.
Zinc configuration. What is zinc's electron configuration? Electronic configuration of the zinc atom in ascending order of the levels: What is the ground state electron configuration for zinc?
Electron configuration of oxygen (o) [he] 2s 2 2p 4: Electron configuration of nitrogen (n) [he] 2s 2 2p 3: Electron configuration and oxidation states of zinc.
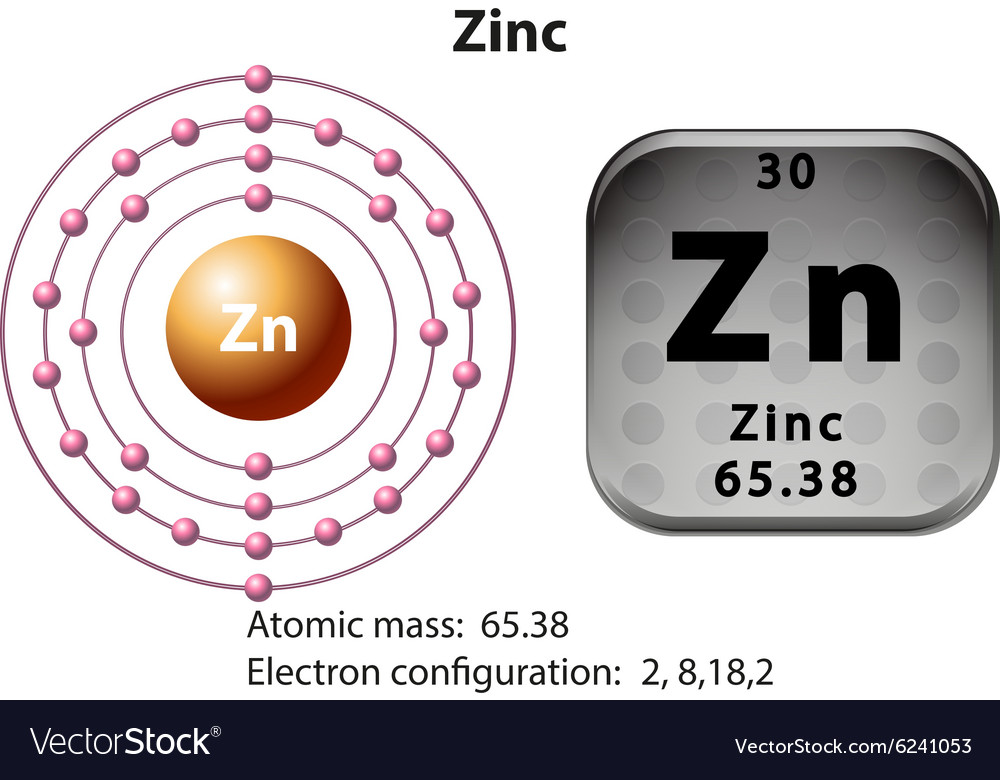
The electron configuration for zinc is 1s2 2s2 2p6 3s2 3p6 4s2 3d10. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 >> back to key information about the elementback to key information about the element Full electron configuration of zinc:
[ar] 3d 10 4s 2 below is the electronic diagram. Electron configuration of zinc is [ar] 3d10 4s2. The electron configurations of the zinc atoms are shown in figure 1.
Possible oxidation states are +2. Here, the electron configuration of zinc ion is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10. In the zinc blende structure, the selenide atoms pack in a cubic symmetry, and the zinc atoms occupy half of the tetrahedral holes, while in the wurtzite structure although the.
Compounds of zinc are chemical compounds containing the element zinc which is a member of the group 12 of the periodic table.the oxidation state of zinc in most compounds is the group. Chemistry electron configuration electron configuration 1 answer anor277 may 24, 2016 1s22s22p63s23p64s23d10 explanation: This electron configuration shows that zinc ion (zn 2+) has three shells and the last shell has eighteen.









