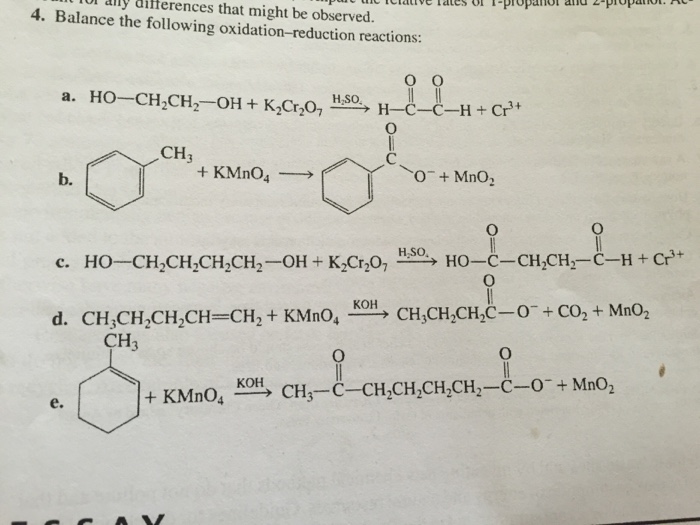
Determine whether the following is an oxidation or reduction reaction.
Which of the following is an oxidation reduction reaction. The reaction is as follows: 2na (s) + cl 2 (g) → 2nacl the reactants are elements, and it is assumed that they are electrically. Ch3ch2oh cro3 ch3ch h2c=chcch, + nh3 h2nch2ch,öch3 > br 1.
Please just write whether it’s an oxidation or. Ch4(g) + 2o2(g) =co2(g) + 2h2o(l) is this an oxidation or reduction. So we write the individual reactions with.
Classify each reaction as oxidation, reduction, or neither. Determine if the reaction is a combustion reaction. There are rules for assigning.
Redox reactions involve formal electron transfer and a formal change in oxidation number. Since the zinc is losing electrons in the reaction, it is being oxidized. The sulfur is gaining electrons and is thus being reduced.
Another example of a redox. Answer the only b is a redox reaction. Oxidation numbers are used to keep track of electrons in atoms.









