Pure water always consists of two hydrogen and one oxygen atom.
Which of the following is a pure substance. Participate in poll, choose your answer. The characteristics of pure substances are: Sodium chloride is a pure substance because it has fixed.
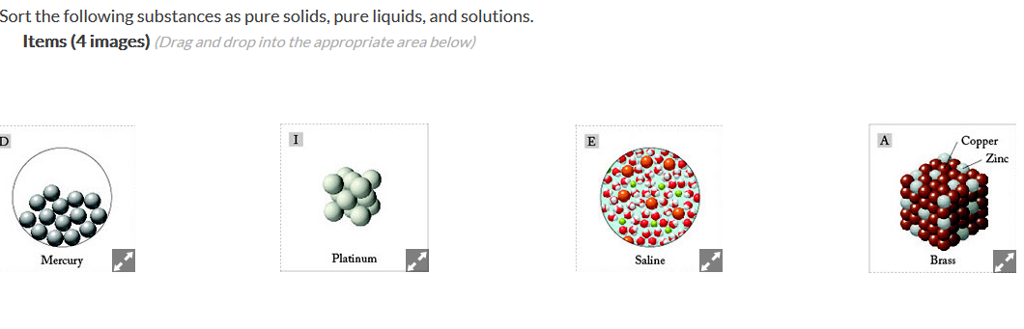
Carbon dioxide is a compound while oxygen and zinc are elements. Examples of pure substances include elements and compounds. Pure substances are mostly homogeneous in nature containing only one type of atom or molecule.
Orange juice with pulp is an. The pure substance formed, n a c l, is formed through electrostatic interactions, not the much stronger chemical bonds of covalently bonded atoms that form molecules. Which of the following is a property of a pure substance?
Zaneslayer2 zaneslayer2 10/01/2018 social studies middle school answered which. Also pure gold consists of gold atoms only. A pure substance is a sample of matter with both definite and constant composition with distinct chemical properties.
These particles are similar to one another and cannot be separated into. Participate in poll, choose your answer. Brainly user brainly user 01/04/2016 chemistry middle school answered which of the following is a.
An element is an example of a pure substance because it cannot be broken down into simpler substance. These atoms are chemically bonded to each other. Pure substances cannot be exemplified by all elements other than nickel because pure nickel is an.









