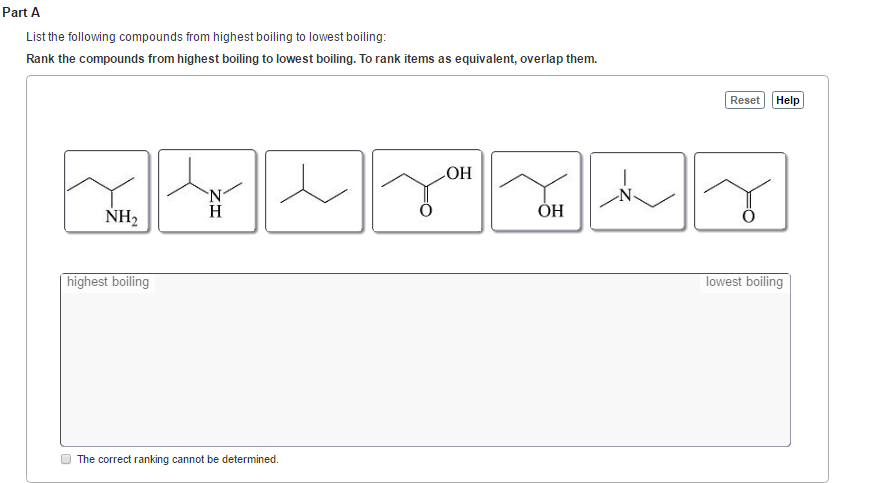
Which of the following compounds most likely have the highest boiling point?
Which of the following compounds would have the highest boiling point?. A) ch_3 ch_2 ch_3 ch_2 ch_5 b) ch_3 ch_2 ch_3 ch_2 oh c) ch_3 ch_2 ch_2 och_3 d) ch_3 ch_2 ch_2 cl. C5h12 1 i advertisement answer 4.0 /5 15 azermacas answer: Expert answer 100% (1 rating) alcohols are generally have high boiling point because of the strong hydrogen bonding with more electronegative oxygen.
Organic chemistry with a biological emphasis by tim soderberg (university of minnesota, morris). Which one of the following compounds will have the highest boiling point? Arrange the following compounds in order of decreasing boiling point.
Which of the following chemical compounds has the highest boiling point? Which of the following compounds would have the highest boiling point? Show transcribed image text expert answer among the following compounds, the compound 4 will have the highest.
Water will have highest boiling point as it forms strong intermolecular hydrogen bonds. Solved which of the following compounds would have the | chegg.com. Among the given options (c) ethanol has the highest boiling point as it has the strongest intermolecular force of hydrogen.
Which of the following compounds will have the highest boiling point? Answer which of the following has the highest boiling point? What compounds have the highest boiling point?
Where as amines generally have. The order of the boiling points are: Which of the following compounds would have the highest.









