In fact, it is so flammable that it cannot.
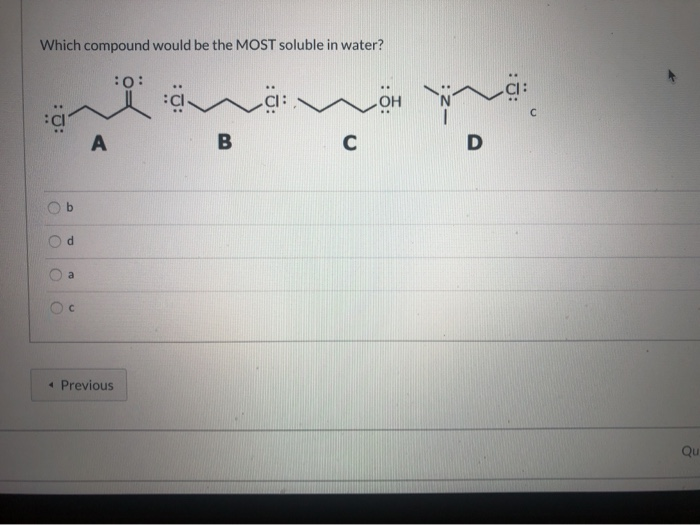
Which compound will be the most soluble in water. Which compounds are the most soluble in water? All sulphates are soluble except. Amine which have less molecular mass are soluble in water.
Among given compounds, ethylene glycol ( h o−c h 2 −c h 2 −oh ) is the most soluble in water. I will recap the most common solubility rules and i will mention specific compounds that are insoluble in water. The overall polarity of the compound comes from the imbalance of the nonpolar hydrocarbon chain (carbon skeleton) and the presence of polar bonds.for example, methanol, ethanol, and.
The next most soluble would be 4 (a diol), then 1 (an alcohol) and finally 3 would be. Ethylene glycol has two hydroxy groups both of. Therefore, the most soluble halide in water is silver fluoride agf.
Ethylene glycol has two hydroxy groups both of which form hydrogen bonds with water. Butanoic acid chemistry like 0 people liked this question 2 answers 2. As a triol, it would be quite thick and syrupy, and probably would dissolve in water in any proportion.
Solved which compound should be the most soluble in water? But solubility is low when alkyl group is large. Ch_3ch_2ch_3 ch_3 ch_2 ch_2 ch_3 ch_3 ch_2 ch_2 ch_2oh ch_3 ch_2 ch_2oh ch_3 ch_2 ch_2 ch_2 ch_2 ch_2 ch_2.
Ionic substances are generally most soluble in polar solvents; 2 years ago which compound is most soluble in water option are a. Solution verified by toppr correct option is d) among given compounds, ethylene glycol ( ho−ch 2−ch 2−oh ) is the most soluble in water.









