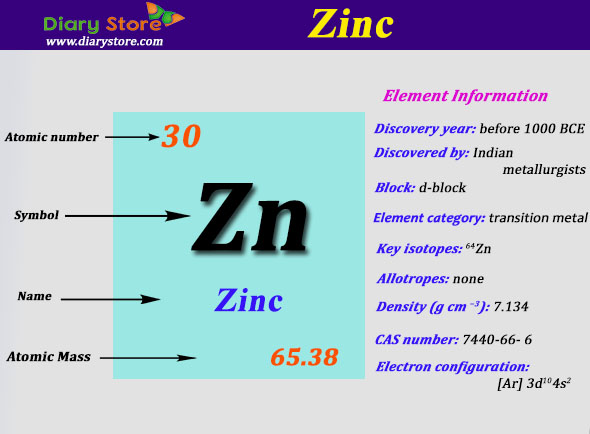
Full electron configuration of zinc:
What is zinc electron configuration. Zn (zinc) is an element with position number 30 in the periodic table. We first need to find the nu. The electron configuration of zinc is 1s22s22p63s23p64s23d10.
The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2. Valence electron configuration of zinc, abbreviated. The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its electronic configuration.
Copper ← zinc → gallium. 5 rows the zinc atom donates two electrons in the 4s orbital to form a zinc ion (zn 2+ ). To write the configuration for the zinc and the zinc ion, first we need to write the electron configuration for just zinc (zn).
What is the electronic configuration of zinc. It is stable in the presence of oxygen, but it is unstable in low oxygen conditions. Electronic configuration of zinc zn:
The 2+ charge denotes that zinc has loosened 2 electrons from its valence shell. What is the outer electron configuration for zinc? A neutral atom has equal numbers of protons and electrons, so a neutral atom of zinc would have 30 electrons.
This means that it has 10 electrons in its outer shell, and that those electrons are in. The full electron configuration of zinc is 1s2 2s22p6 3s23p63d10 4s2zinc, also written zinc, is defined as the chemical element that belongs to the periodic table of elements. The electron configuration of a neutral zinc atom is 1s 22s 22p 63s 23p 63d.








