The deaf pressure that is applied to stop the action of osmosis is called osmotic pressure.
What is osmosis example. It is a process used in extracting a large number of contaminants from water by pushing the water under pressure through the membrane. When your hands are immersed in dishwater for a long time, your skin looks bloated. A good example of osmosis is seen when red blood cells are placed into fresh water.
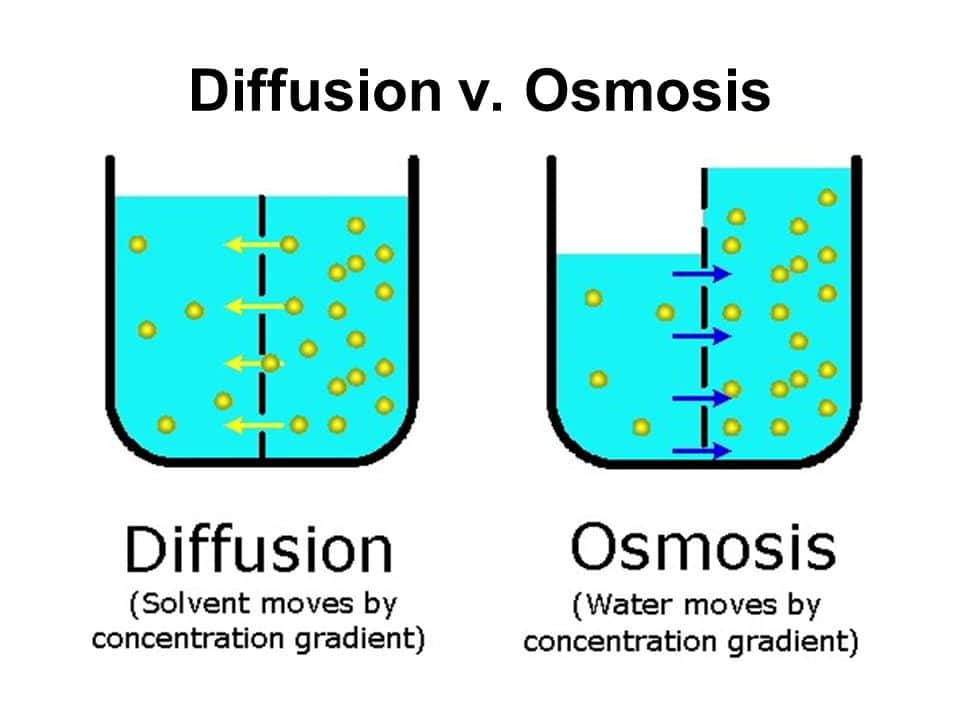
Osmosis is an example of diffusion , which is when molecules tend to. When you pour salt onto a slug, water diffuses. Osmosis ensures that the molecules of wastes as well as excess water in.
The absorption of water by the roots in plant. The process of osmosis concerns the flow of a solvent, such as water, through a semipermeable membrane. Another classic example of osmosis in plants is the swelling up and shrinking of potato cells when slices of potato are dipped in a hypotonic solution and hypertonic solutions.
One of the best examples of osmosis is seen in the kidneys. The greater the surface area, the faster the rate. A common osmosis example seen in real life is the preservation of food.
Osmosis examples in daily life. Water is transported by vascular tissues xylem where the nonliving tracheid are also taking up the water. Examples of the osmosis process.
When a plant cell is filled with water the guard cells swell up. If you want to understand osmosis you need to understand first diffusion since osmosis is a type of diffusion diffusion is the movement of molecules from high concentration. Unicellular living beings that live in fresh water enter large amounts of water through osmosis.









