Web there are two ways to find the number of valence electrons in neon (ne).
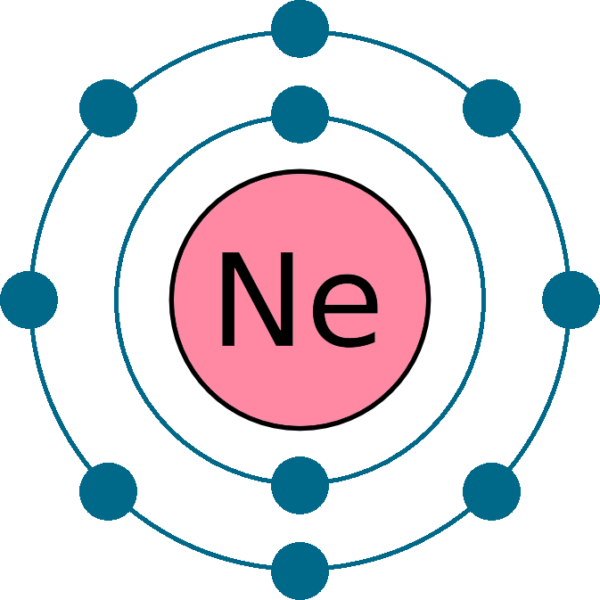
What is neon valence electrons. Web the valency(valence) of a neon atom is zero and the valence electrons of a neon atom are 8. The total number of electrons in the last shell after the electron configuration of neonis called the valence electrons of neon. Web a valence electron is the total number of electrons found in a valenceshell.
Electronegativity (pauling scale) the tendency of an atom to. Web in fact, the number of valence electrons goes up by one for each step across a period, until the last element is reached. Now let’s see how you can easily find the.
Valence electrons for transition elements. Each element has different number of. Remember that an element's electron.
The valence electron is the total number of electrons in the last orbit(shell). Web valence electrons are the electrons in the outermost shell, or energy level, of an atom. The first is to use the periodic table to figure out how many electrons neon has in its.
Transition elements are a bit trickier. Web neon has 8 valence electrons because there are 8 electrons present in the outermost shell of the neon (ne) atom. In the diagram, the 2s orbit has 2 valence electrons where the 2p orbit has other six valence.
This outermost shell is known as. Web the number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. Web when i want to figure out how many valence electrons sodium has, the number of valence electrons would be equal to the number of electrons in the outermost shell, the.






![[5 Steps] Electron Configuration for or of Neon in Just 5 Steps](https://i2.wp.com/2.bp.blogspot.com/-jMs9FCVMeSw/XD4FtZiSVlI/AAAAAAAAYZg/rJnlLKM6S6EHv8FmJZr4pj2PmzTZjk0hQCLcBGAs/s1600/20190115_220744.jpg)
