Web we would like to show you a description here but the site won’t allow us.
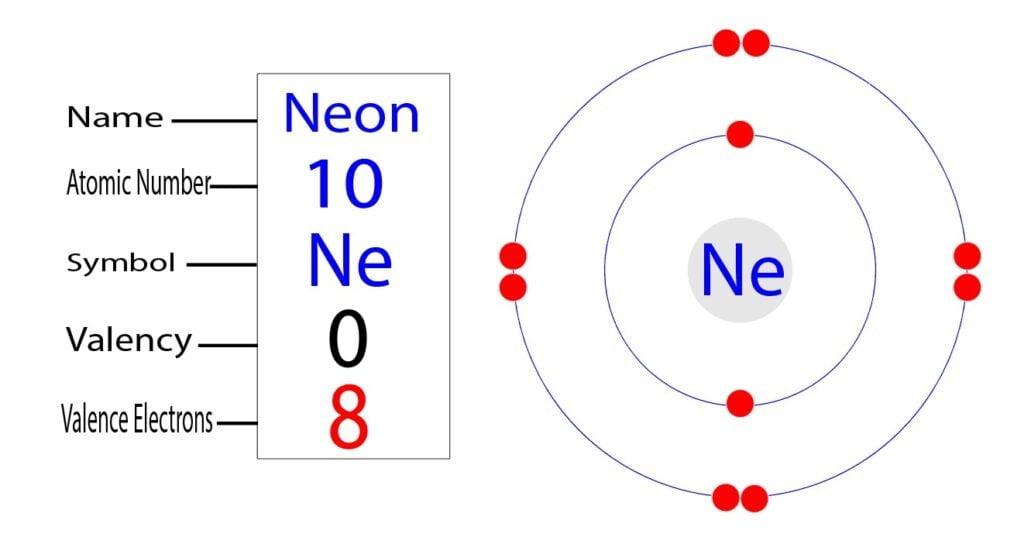
What is neon's mass number. It is element #10 in the periodical. Stable isotopes of neon are produced in stars. Neon, ne, is a noble gas and it is a gas that fills neon signs.
So, the number of neutrons in the neon atom is 10. Web so let me move down here so we can look at the definition for the mass number. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images.
Web chemical element, neon, information from authoritative sources. 1 become a study.com member to unlock this answer! Neon is a chemical element with atomic number 10 which means there are 10 protons and 10 electrons in the atomic structure.
The chemical symbol for neon is ne. This requires temperatures above 500 megakelvins, which occur in the cores of stars of more than 8 solar masses. Web neon has an atomic mass of about 20 this isotope contains 10 protons and 10 neutrons.
Neon is abundant on a universal scale; The mass number is the total number. Web the chemical symbol for neon is ne.
Consider the table below, which shows. Mass numbers of typical isotopes of neon are 20; Web the mass number (represented by the letter a) is defined as the total number of protons and neutrons in an atom.






