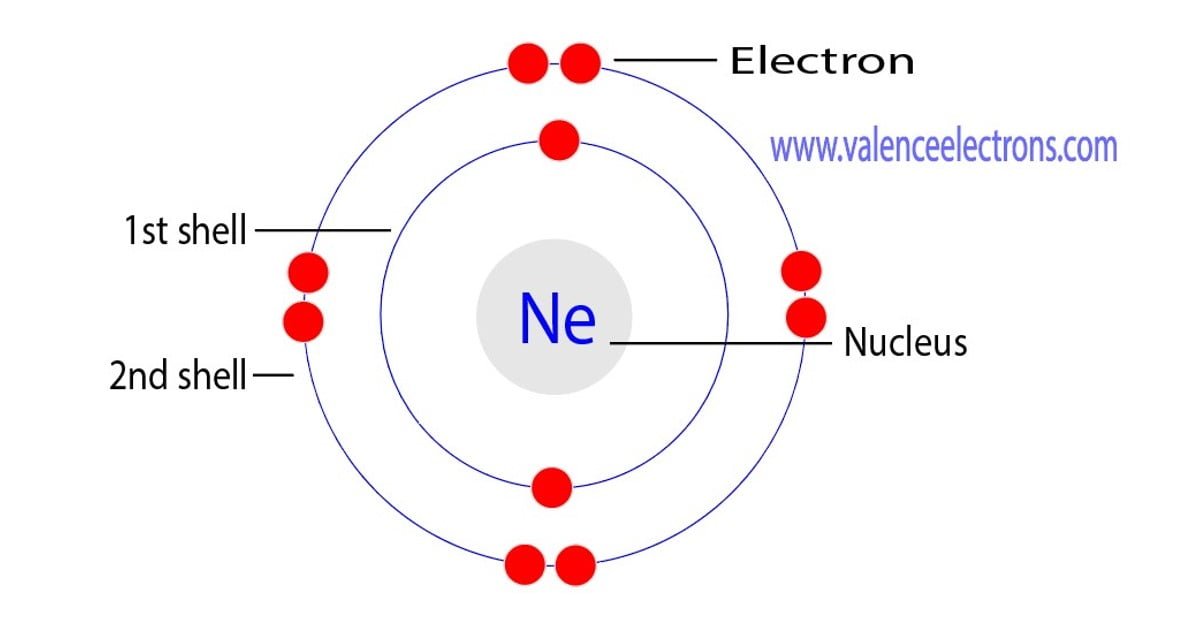
We can make an electronic configuration of an element, which is known as the atom and today in this topic we are going to discuss the electron configuration of neon atom.
What is neon electron configuration. For example, to find the configuration for the lithium ion (li⁺), start with neutral lithium (1s²2s¹). Web for example, [ne] represents the 1s 2 2s 2 2p 6 electron configuration of neon (z = 10), so the electron configuration of sodium, with z = 11, which is 1s 2 2s 2 2p 6 3s 1, is written as [ne]3s 1: So, the electron configuration of neon is 1 s 2 2 s 2 2 p 6 now, the neon atom have fully filled valence shell.
Possible oxidation states are 0. [he] 2s 2 2p 6. The distribution of electrons are as 2 electrons in 1s subshell, 2 electrons in.
Web electronic configuration of neon (ne): The abbreviated electronic configuration of neon is [he] 2s2 2p6. Web in atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals.
For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s and 2p subshells are occupied by 2, 2 and 6 electrons. We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): Web 【】what is the neon (ne) electron configuration?
Calculate the maximum number of electrons each subshell can hold using the formula: Web the electronic configuration of neon is ne: The number of the principal quantum shell, n, the letter that designates the orbital type (the subshell, l), and
Web electron configuration of neon is [he] 2s2 2p6. Since the atomic number of neon is 10, the total electrons of neon are 10. Web neon electron configuration:
![[5 Steps] Electron Configuration for or of Neon in Just 5 Steps](https://i2.wp.com/2.bp.blogspot.com/-jMs9FCVMeSw/XD4FtZiSVlI/AAAAAAAAYZg/rJnlLKM6S6EHv8FmJZr4pj2PmzTZjk0hQCLcBGAs/s1600/20190115_220744.jpg)






