How do you recognize electron configuration?
What is energy level in electron configuration. Basically the distribution of electrons over various shells (energy. It states that, in the ground state, the electrons occupy the atomic orbitals in. Two of the lithium electrons can.
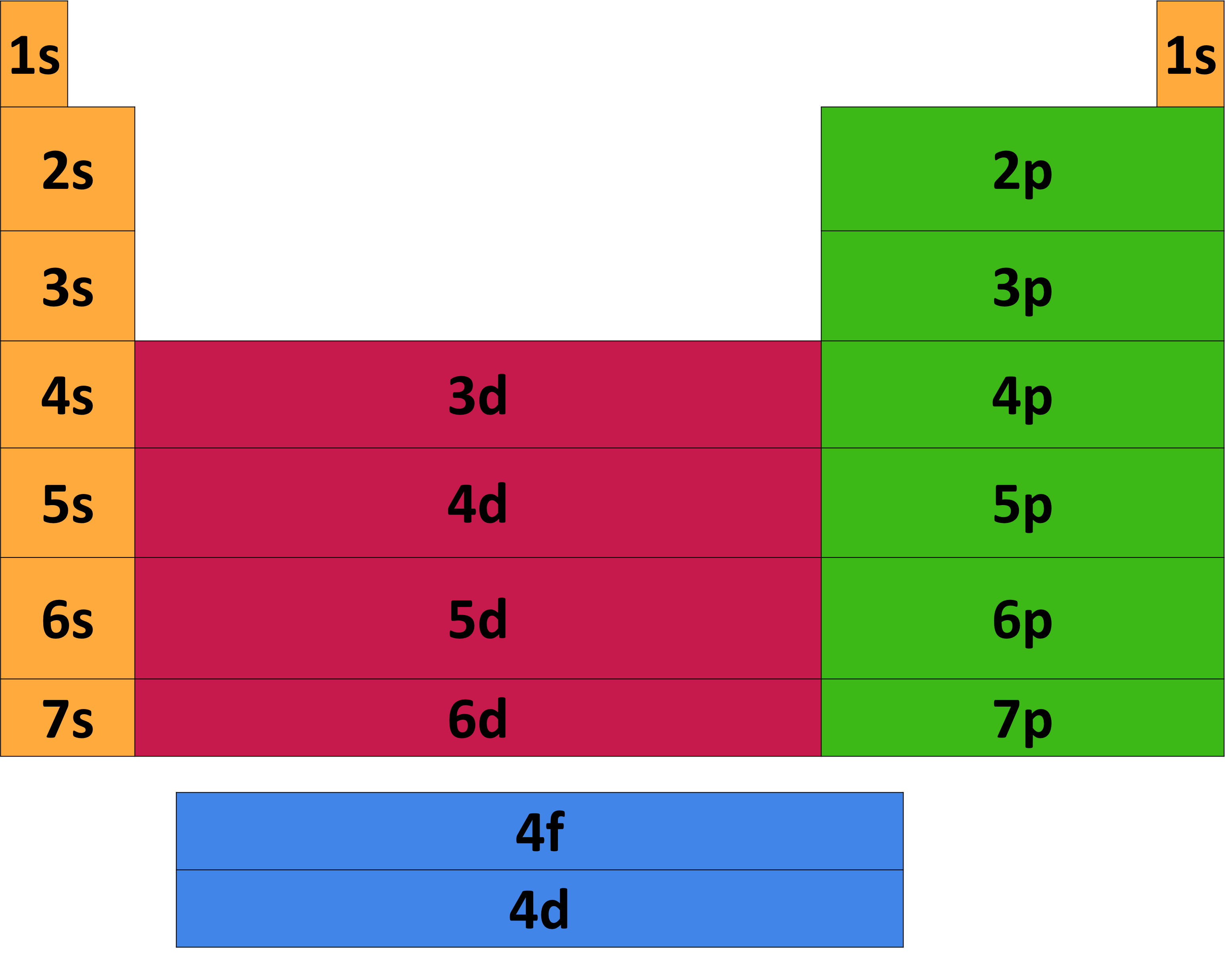
Learn the basics about energy levels and electron configuration. The systematic distribution of electrons in the various atomic orbitals is called its electronic configuration.in the electronic. Electron configuration was first conceived under the bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the.
According to this scheme, the periodic table can be divided into s,. The electrons are distributed over different energy level. Electron configuration is the representation of how the electrons in an atom are arranged, which can be used to predict the properties of an element.
The highest atomic orbitals occupied by electrons determine the properties of the elements. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found.as you go farther from the nucleus electrons at higher. (2 electrons in the first energy level and 1 electron in the second energy level) for atoms with more than 3 electrons,.
An atom's electron configuration is the way in which its electrons are distributed among its various orbitals. However, one can write the electronic configuration just by understanding the aufbau principle. The electronic configuration of n2 is kk (σ(2s)) 2 (σ ∗ (2s)) 2 (π(2p x)) 2 (π(2p y)) 2 (σ(2p z)) 2.
N b = 8, na= 2. 119 rows shorthand electron configuration full electron configuration electron shell arrangement; It is expressed by ‘l’.





/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)



