The solubility of the gas.
What is diffusion what are the factors on which rate of diffusion depends. For nutrients to diffuse into a cell they must traverse the cell membrane. Diffusion takes place as long as there is a difference between the concentrations of a. The smaller the molecule such as gas, the faster the rate of diffusion while the larger the molecules (liquid) the.
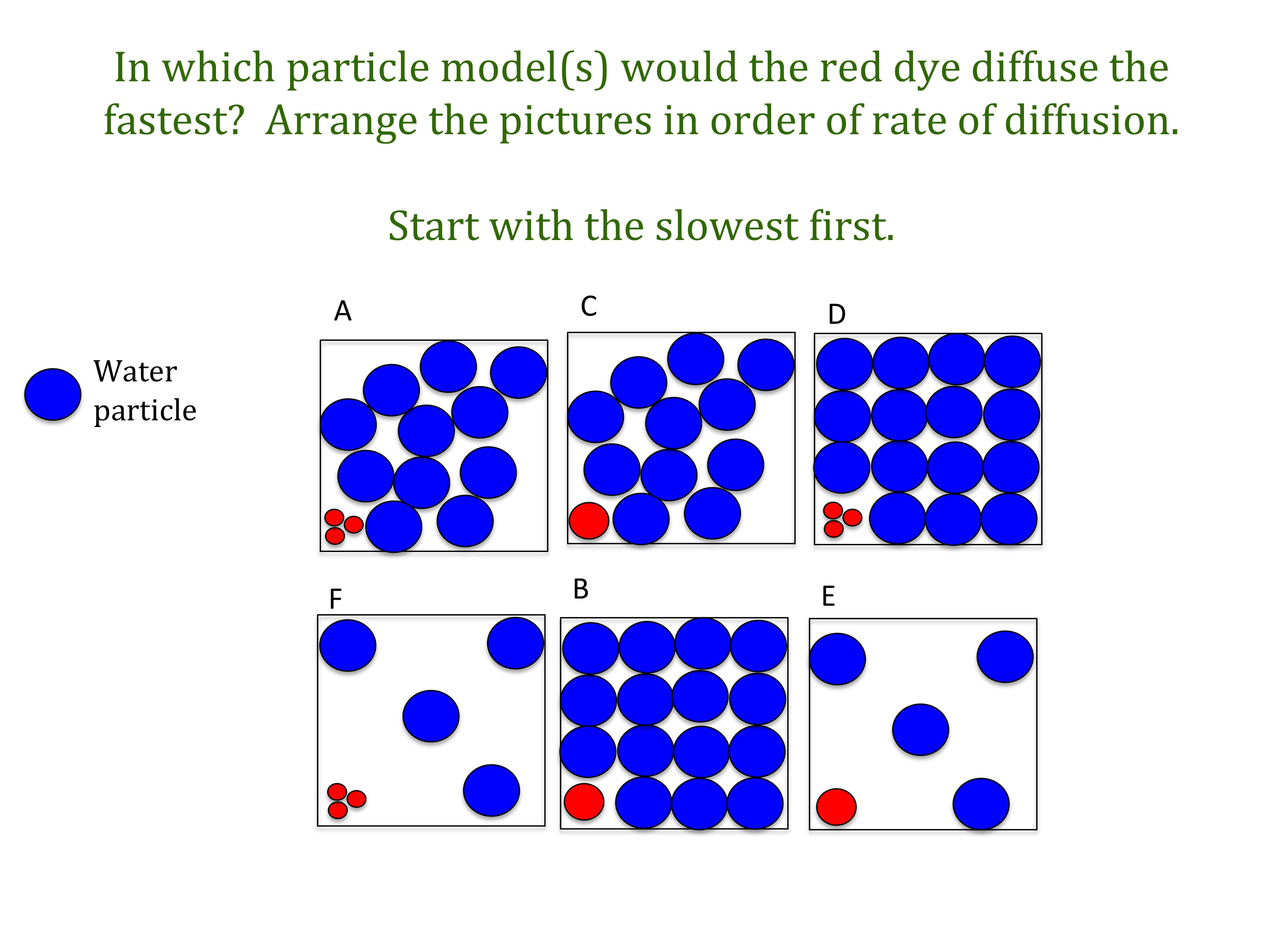
The rate of diffusion is affected by the concentration gradient, membrane permeability, temperature, and pressure. What factors affect the rate of diffusion? Diffusion rates are affected by the permeability of the membrane separating them, concentration gradient, pressure and temperature.
The partial pressure difference across the diffusion barrier. What is diffusion and how the rate of diffusion depends on density and write some applications of diffusion. Medium solution verified by toppr was this answer helpful?
Several factors influencing the rate of diffusion of a solute comprise the mass of the solute, the atmosphere temperature, the density of solvent, and the distance travelled. These are the factors that affect rate of diffusion; Diffusion is the movement of molecules from a region of higher concentration to a region of lower concentration down the concentration gradient.
The rate of diffusion depends on many factors like the concentration gradient, equilibrium of the system, temperature, mass of the particle, and many more. Fick's law gives us a number of factors that affect the diffusion rate of a gas through fluid: Rate of diffusion is affected by;
The rate of diffusion depends on three factors: The rate of diffusion depends on the difference between concentrations across the host material, with higher concentration differences resulting in higher diffusion rates. Diffusion takes place as long as there is a difference between the.









