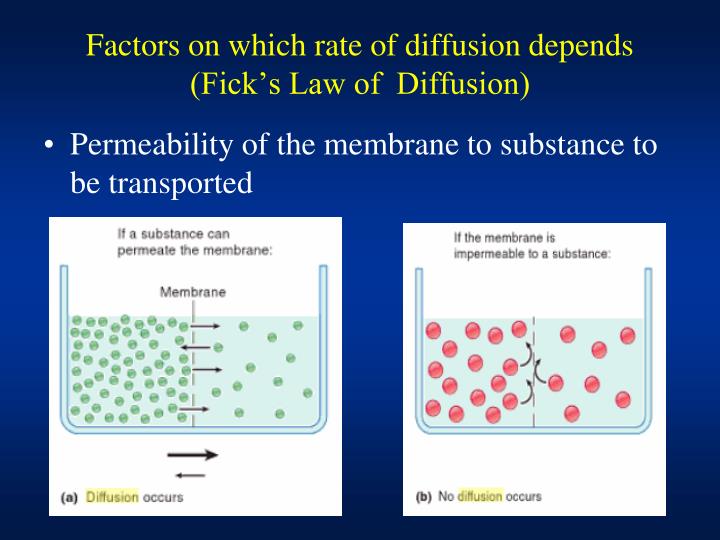
The concentration gradient (the increase or decrease in concentration from one point to another);
Rate of diffusion depends on. 1) put the gas in a closed container and weigh it on a scale. An example of this is the way. Thickness of the diffusion surface.
The rate of diffusion depends on the difference between concentrations across the host material, with higher concentration differences resulting in higher diffusion rates. Diffusion of any substance across the cell membrane also depends upon it's solubility in lipids. The rate of diffusion depends on three factors:
Factors that affect rate of diffusion the size of the molecule: The smaller the molecule such as gas, the faster the rate of diffusion while the larger the. Gases dont diffuse at the same rate every time.
The amount of surface area available for. The greater the density, lower the rate of diffusion. The mass of the particles.
Igcse chemistry mr.richard by save and karan 10s. The diffusion rate depends on several factors: A small pin hole is made into container.
The rate of diffusion depends on the mass of matter. The diffusion rate depends on the size of the substances and smaller substances diffuse faster. Lighter particles will diffuse at a faster rate.









