Honoring scientists from early career to expert level chemists.
Periodic table with energy levels. Watch an overview of lesson 4.3 featuring an introduction to the energy levels in an atom for the first 20 elements. It is the pauli exclusion principle that requires the electrons in an atom to occupy different. 119 rows chemical elements listed by ionization energy the elements of the periodic table sorted by ionization energy.
Tabular layout of the chemical elements in order of atomic number this article is about the table used in chemistry and physics. After the third energy level has 8 electrons (argon), the next 2 electrons go into the fourth energy level. For instance, lithium () has three electrons:
Ionization energy of nitrogen (n) 14.53 ev. Two fill the orbital, and the third is placed in. The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements.
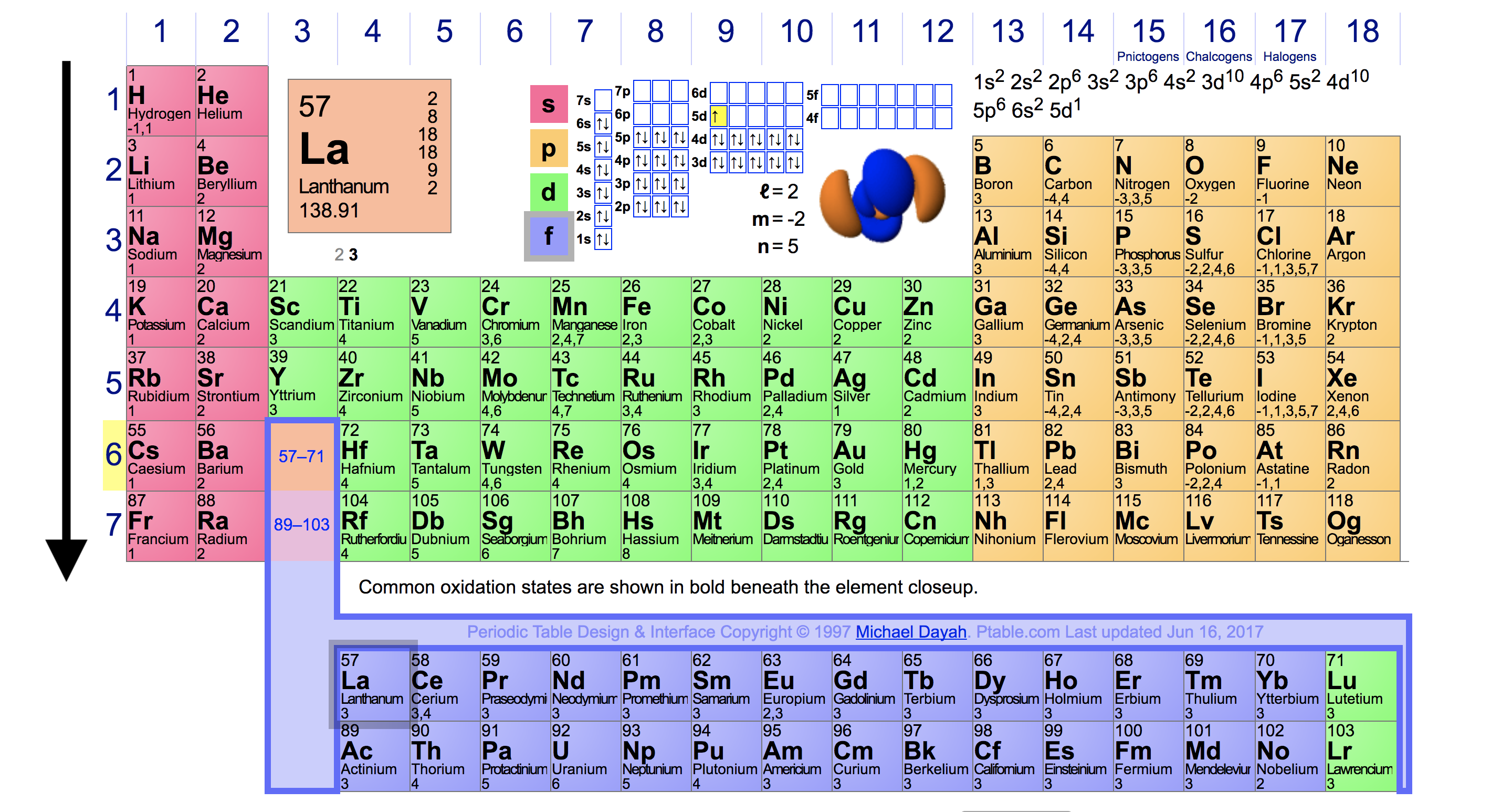
Interactive periodic table showing names, electrons, and oxidation states. The periodic table is arranged so that atoms with the same distributions of valence electrons are arranged in columns. An energy level model is shown in the chart.
The electronic configuration of each element is decided by the aufbau principle which states that the electrons fill orbitals in order of increasing energy levels. In the periodic table, the elements are listed in order of increasing atomic number z. Elements in the second row of the periodic table place their electrons in the 2n shell as well as the 1n shell.
The 5g 6f and 7d orbitals should have about the same. The energy level periodic table is a collection of formulas that help people understand how energy moves about the earth. Click on any element's name for further information on chemical.









