As with ir spectroscopy, nmr spectroscopy passes light through a sample and looks at the spectrum that is transmitted.
How to read nmr and ir. Singlet (3h) at ~2.4 ppm. Quartet (2h) at ~4.3 ppm. Chemists typically use infrared spectroscopy to.
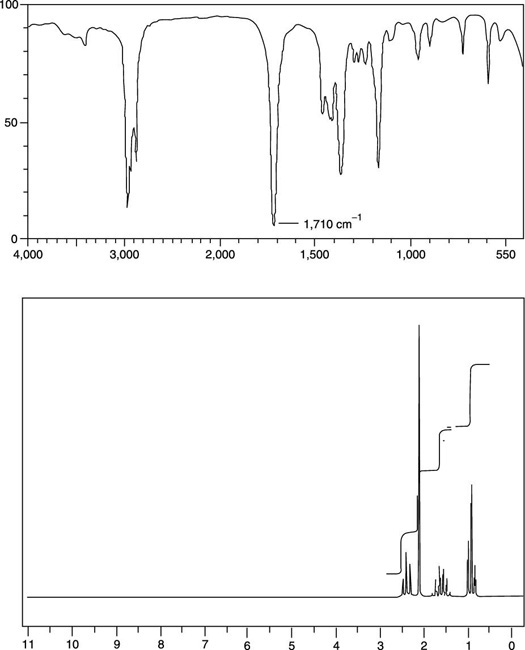
We can use spectroscopy to determine the structure and functional groups in organic compounds. (λmax) is read directly from the spectrum and the molar extinction. Spectroscopy is the study of how light interacts with matter.
The videos below will take you through. In this video i use the molecular formula, ir spectrum, and nmr spectrum to determine the organic structure Nuclear magnetic resonance (nmr) spectroscopy uses the magnetic properties of nuclei to discover the properties of the nuclei's parent atom.
For the compound with the structure given, we. Infrared radiation causes a vibrational transition in a given molecule. The two doublets lend to a disubstituted benzene.
How to read nmr and ir. In this case, however, absorption occurs at frequencies. We will be learning about.
The chemical shift, multiplicity, coupling constants, and integration are all factors to. Introduction to ir and nmr spectroscopy part 2: Doublet (2h) at ~7.2 ppm.