The attraction created by hydrogen bonds keeps water liquid over a wider range of temperature than is found for any other molecule its size.
How to draw water molecules. Web we would like to show you a description here but the site won’t allow us. The water extends highest where it contacts the edges of the tube, and dips lowest in the middle. Web the water molecules are more strongly attracted to the glass than they are to other water molecules (because glass molecules are even more polar than water molecules).
This causes the hydrogen atoms to bond at about a. A water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Web take the softest pencil and accentuate some parts of the streams, especially in the shadow and next to dark parts that could be reflected by water.
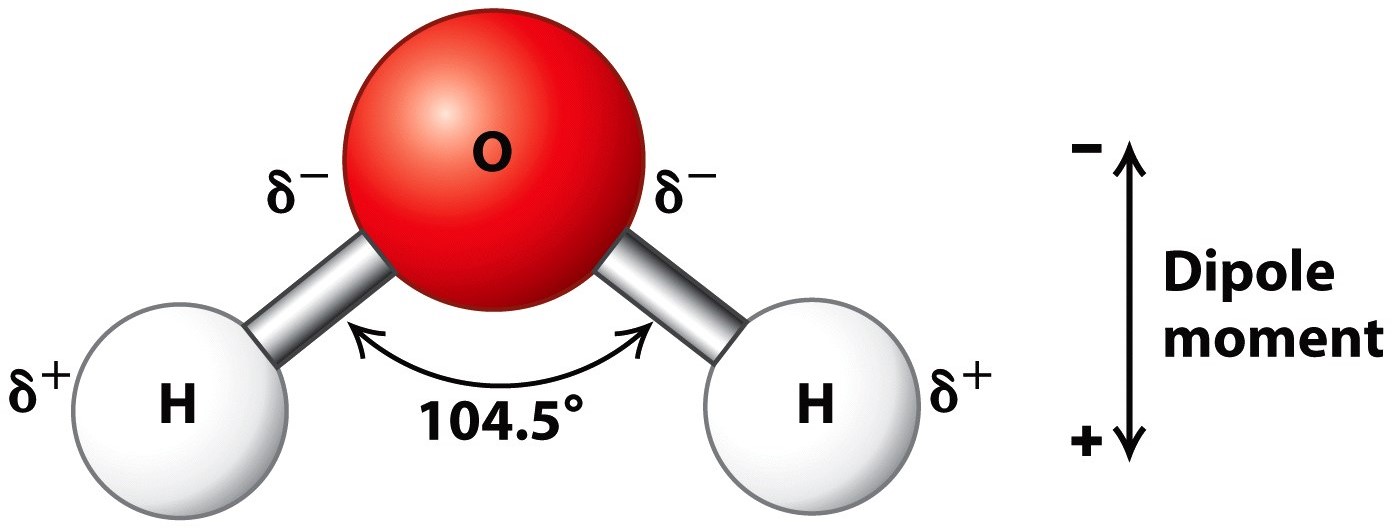
The surface molecule is attracted to its neighbors below and to either side, but there are no attractions pointing in. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). This means that ice is less dense than liquid water, which is why it floats.
You can either type in “o”, or select “oxygen (8)” from the drop down menu, and then click on the black display. So, water molecules are able to form hydrogen bonds with one another, giving water many of its unique properties. Web the key to understanding water’s chemical behavior is its molecular structure.
Then we'll look at the π mos for the nitrate ion, so we can see the difference between mo theory and valence bond theory. In a water molecule, an oxygen atom forms two bonds, one to each hydrogen atom. Atoms can form more than one bond.
You can see this by looking at the image below: During this course, you will view molecules written in all three forms. Take a hard pencil again and sketch the foam under the waterfall.



















