An example of such an.
How to draw the surface tension of water. The paper clip will sink as the water can no longer support the weight of the paper. Surface tension can be defined as σ = fs / l (1). Web how to draw surface tension of water and diameter of bore diagram of class 11.
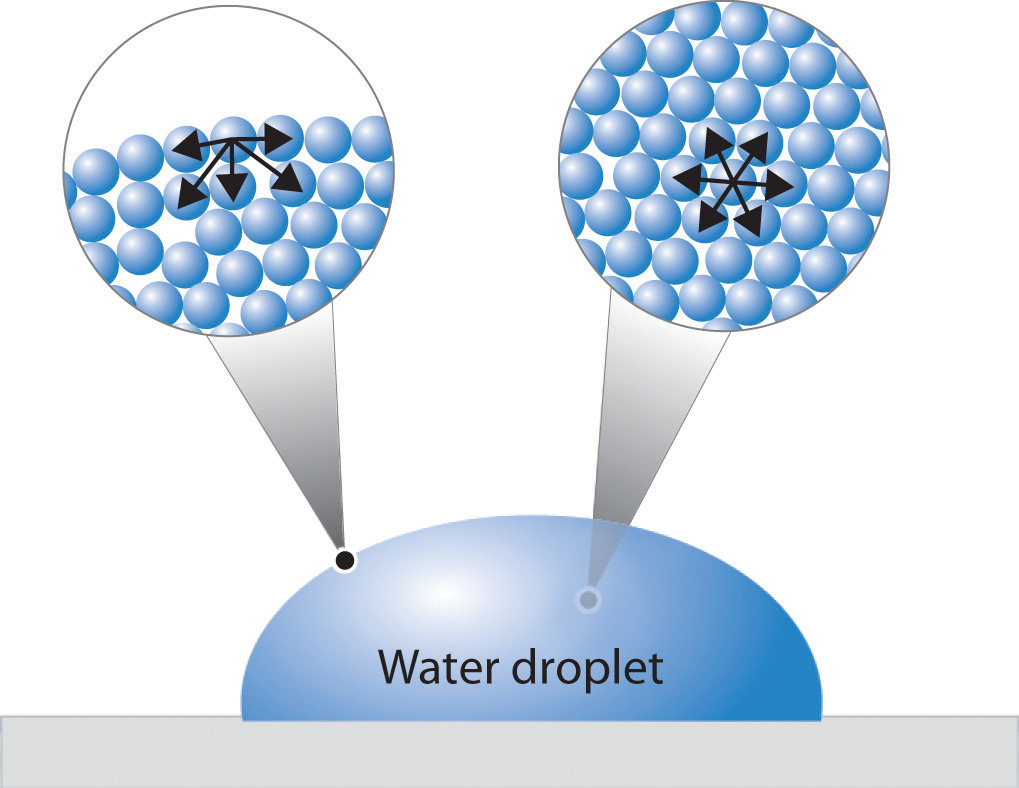
Web at the surface of water, molecules are more densely packed because they are not being pulled from above, resulting in stronger intermolecular forces. Web 94,331 what causes surface tension? Now that you know how much external force the.
This creates surface tension, which allows for phenomena such as water droplets maintaining a round. Web surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure \(\pageindex{2}\) ). Along the surface, the particles are pulled toward the.
Web liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. Surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface tension performs. Along the surface, the particles are.
The surface of the water is made up of millions of water molecules. Web water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Web causes of surface tension.
Water molecules on the surface of. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Web the explanation involves raoult's law, hydrogen bonding, adsorption, and surface tension, so this phenomenon makes a good review of much you have learned.



















