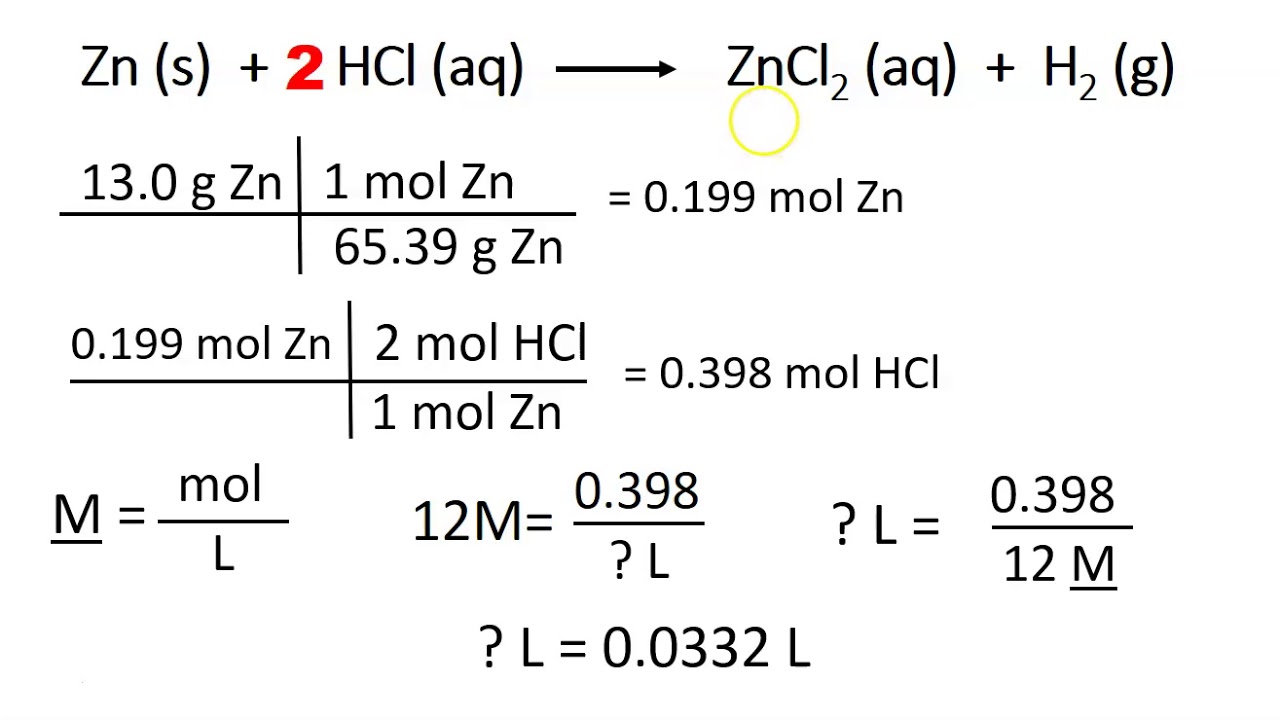
Write the balanced chemical equation.
How to do stoichiometry problems. Look at the problem and figure out. Use the mole ratio to. There are four steps in solving a stoichiometry problem:
It is important to remember that solving. Convert the units of the given substance (a) to moles. A common type of stoichiometric relationship is the mole ratio, which relates the amounts in moles of any two substances in a chemical reaction.
Use the mole ratio to calculate. 5 simple steps to solve solution stoichiometry problems. Write the balanced chemical equation.
To make it easier to solve stoichiometry problems arrange the equation so it looks like this pv/8.314*k. Show all work and consider. Always have a balanced chemical equation!
Let’s say that we have this equation, which is the combustion of. What mass of fe 2 s 3 is produced when 4.68 grams of fe reacts with 2.88 grams of s?. There are four steps in solving a stoichiometry problem:
Chemical stoichiometry refers to the quantitative study of the reactants and products involved in a chemical reaction. This college chemistry video tutorial provides plenty of stoichiometry problems for you to work on. First of all, we have to start with a balanced reaction.
.PNG)








