Therefore, 4 moles of h 2 o were produced by.
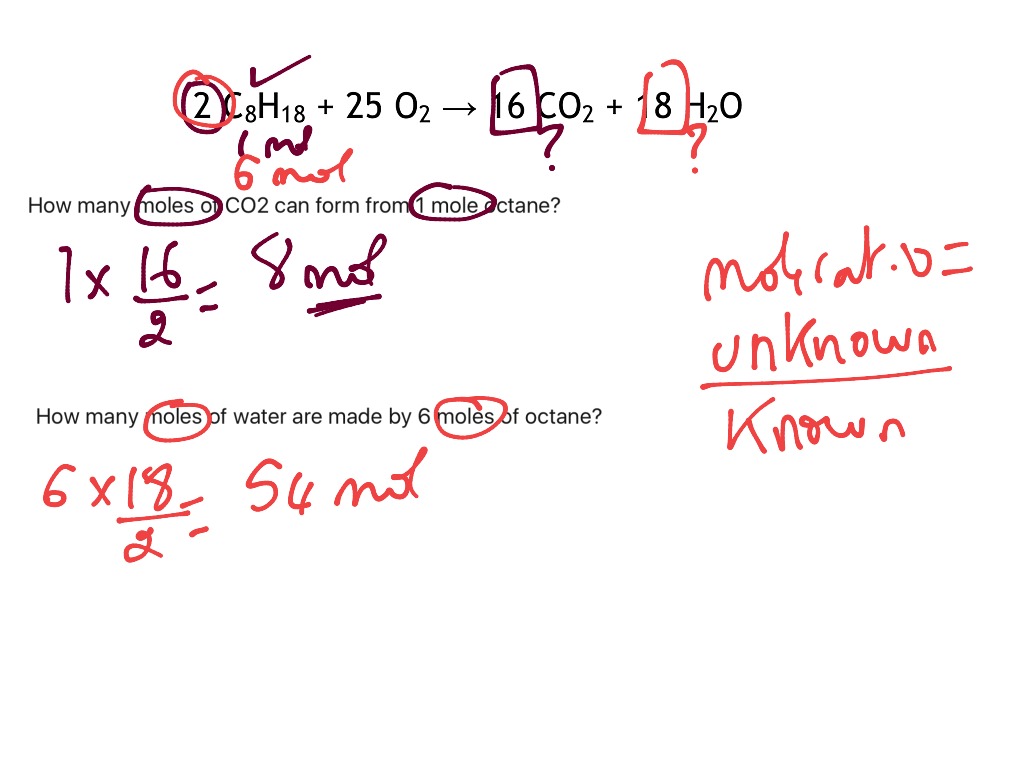
How to do stoichiometry mole to mole. The mole the mole is a key unit in chemistry. How to do stoichiometry calculations ideas by using molar ratio, calculate the moles of the substance which is yielded by reaction. Example, we are given a chemical reaction ch4 + o2 à co2 + h20.
It contains mole to mole conversions, grams to grams and mole to gram dimens. Shows how to use stoichiometry to determine the number of moles of reactants and products if you are given the number of grams of one of the substances in th. This page provides exercises in using chemical reactions to relate moles of two substances.
Calcium, for example, has an atomic mass of 40. There are two ways to solve problems where you are given a balanced equation and asked to predict how many moles of a substance are formed. Carefully adding exact amount of one solution to completely react with another.
The mole and stoichiometry are explained here. In performing mole to mole stoichiometry the first thing we need to do is to balance a chemical reaction. It contains plenty of examples of mole.
Convert the units of the given. Therefore, the ratio is one mole of o 2 to two moles of h 2 o, or. How do we measure the mass of different components in a chemical reaction?
2) every one mole of cuso 4 ⋅ 5h 2 o that is heated releases five moles of water. A common type of stoichiometric relationship is the mole ratio, which relates the amounts in moles of any two substances in a chemical reaction. Assume abundant hydrogen and two moles of o 2, then one can calculate:









.PNG)