Energy levels, electrons, and the periodic table.
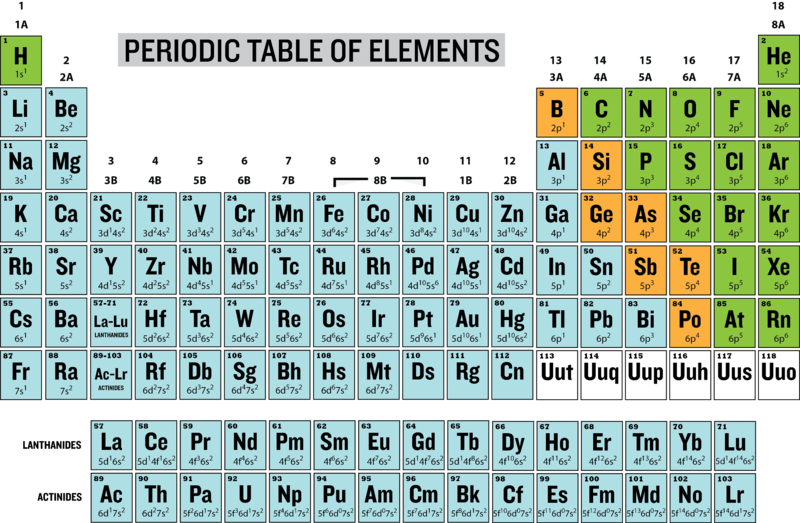
Fourth energy level periodic table. After the third energy level has 8 electrons (argon), the next 2 electrons go into the fourth energy level. The fourth energy level of the periodic table includes the 4s 3d and 4p orbitals. A certain number of electrons go into a level before the.
There is a 4d orbital with 10 electrons which coincides with the. The 4p orbital holds 6 electrons. The first thing you have to know about electron configurations is that there are loads of exceptions, which.
An energy level model is shown in the chart. The fourth energy level has 18 electrons. This is because of the weird way in which atoms fill their sublevels.
The 4s orbital holds 2 electrons the 3d orbital holds 10. See answer (1) best answer. Kitchen meticulously organize your spices in several groupings, be alphabetically or according to the frequency with.
The 4th ionization energy of the element m is a measure of the energy required to remove one electron from one mole of the gaseous ion m. All elements in the 4th period (elements with atomic number 19 to 36) have four energy levels. Third energy level = 1, 2, 3,.8;
The elements in the fourth row of the periodic table.potassium, calcium, scandium, titanium, vanadium, chromium, manganese, iron, cobalt, nickel, copper, zinc. Electrons go into the third energy level. Fourth energy level periodic table human nature to organize things.









