01 51022222233336 11 141231 17 150707 10 A213B1.
Example of udi label. The EU as chair of the International Medical Device Regulators Forum IMDRF working group on UDI strongly contributed to the preparation of the international guidance on a unique device identification system for medical devices which was adopted in December 2013. The UDI documents of the three first designated issuing agencies GS1 HIBCC and ICCBBA are known and were already available on the FDA website. Example of iccbba easily readable plain text udi.
Bear in mind also that sometimes the terminology used here is a little hazy. The GS1 UDI can be created as a GS1-128 or a GS1 DataMatrix depending on space allotment on the label. Our flexible Metalphoto Foil UDI Label has the added benefit of working well on curved or uneven mating surfaces.
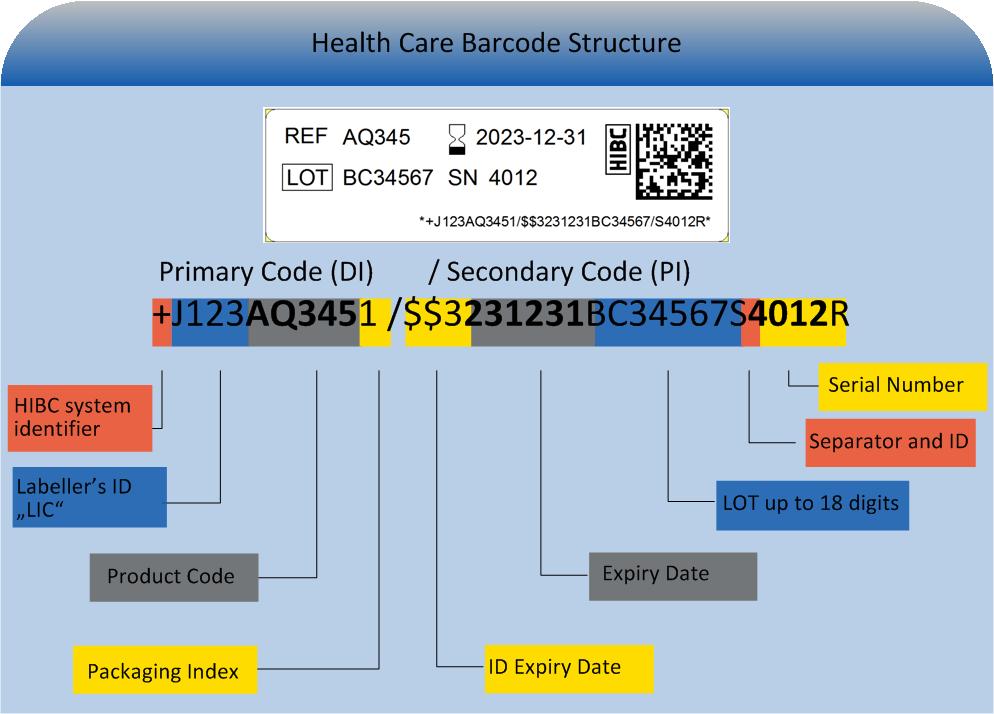
DI not on individual device with Multiple Package Levels. HIBCC UDI Label Examples DataMatrix HIBCC DI Fixed Product Data HIBCC PI Variable Production Data Linear Concatenated HIBCC DI Fixed Product Data HIBCC PI Variable Production Data Linear Not Concatenated HIBCC DI Fixed Product Data HIBCC PI Variable Production Data CA LOT CompuHyper GlobalMed Ultra ImplantableTM Fictitious Medical Device 225 mm x 8 mm 123ABC 1234AB SN USE BY. One of these in mandatory and the other is voluntary.
The UDI itself is made up of two parts. The final human readable string for this example is. Letters and numbers which can be read by people and are encoded in gs1 aidc data carriers confined to gs1s standard structure and format.
7 Type of product certificates. The cartons are often broken down or discarded when the pouches are put into a supply cabinet or on a shelf. The UDI procedure meets FDA Issuing Agency GS1 standards and the requirements of 21 CFR Part 830.
Example of a US compliant UDI label using GS1 standards What are the main difference between the USA and the EU requirements for AIDC. For reusable devices the UDI should be directly marked on the product. A UDI label may be printed directly onto the device itself or appear on packaging.





.png.aspx)












