Other substances that we commonly think of as gases include CO NO HCl O 3 HCN H 2S CO 2 N 2O NO 2 SO 2 NH 3 PH 3 BF 3 SF 6 CH 4 C 2H 6 C 3H 8 C 4H 10 CF 2Cl 2.
Example of gas room temperature. For four examples the mechanical property retentions expressed in percentage of the room temperature values depend on the reinforcement varying between. Molar Volume of Gas. Just Now Chemfsuedu Get All.
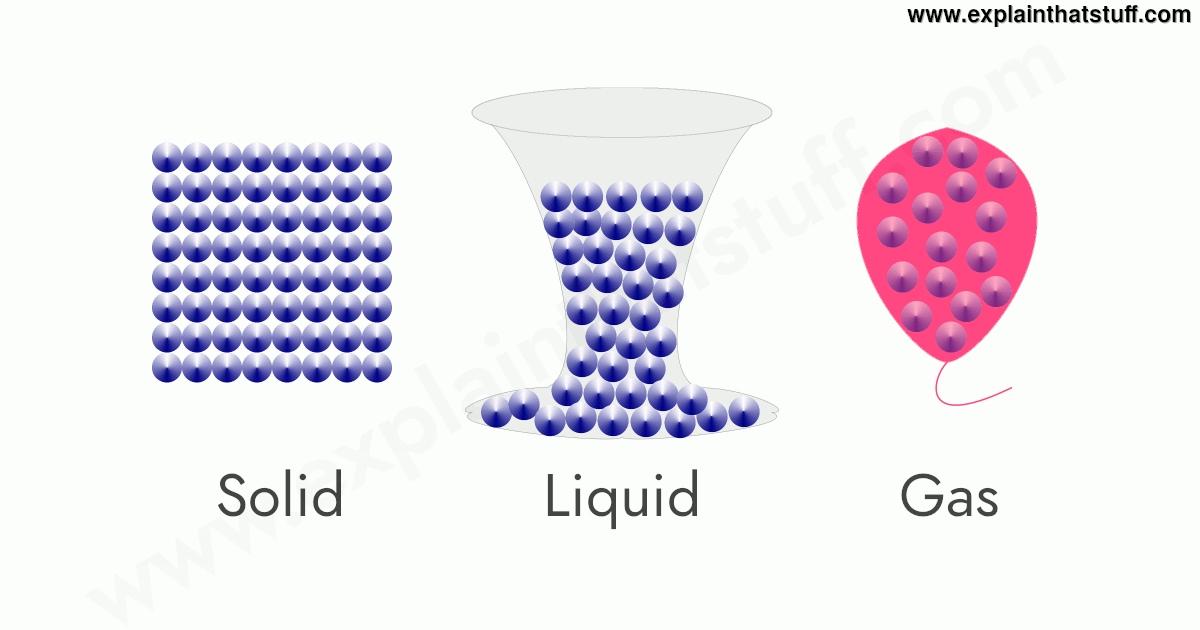
Some examples of pure gases and mixed gases include. One example of a substance that shows covalent network bonding is diamond Figure 82 Diamond which is a form of pure carbon. Click any element below to see all the samples of that element.
Examples of glass transition temperature versus water content. While these substances are all gases at room temperature and. At room temperature 25ºC and pressure 1 atm one mole of any gas occupies a volume of 24 dm³ 24 000 cm³ At standard temperature 0ºC and pressure 1 atm one mole of any gas occupies a.
At temperatures over 3500C diamond finally vaporizes into gas-phase atoms. 1 shows how hot gas from a furnace cools from 300C to 30C in the time it takes to travel through about 120 mm of bare tubing. One mole of a dilute diatomic gas occupying a volume of 1000 L expands against a constant pressure of 2000 atm when it is slowly heated.
Even when the tubing is insulated the gas cools to the ambient temperature after. Gas at Room Temperature 13 These elements are gasses at room temperature and pressure. It can be noted that these substances exist in the gaseous phase under standard conditions for temperature and pressure STP.
And similarly the baked bread that comes out of a bread oven is also roughly 50 greater than the room temperature dough that goes in. Following formula shows ratio of diffusion rates of two gases at same temperature. Gases have different diffusion rates at different temperatures.