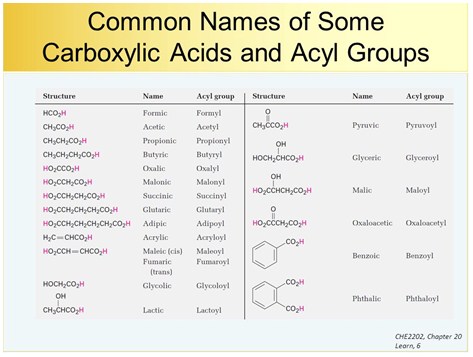
In both common and International Union of Pure and Applied Chemistry IUPAC nomenclature the ic ending of the parent acid is replaced by the suffix ate Table 153 Nomenclature of Esters.
Ester name ending. The simplest esters are those where both R 1 and R 2 are an alkane less the hydrogen atom at the end of the chain and hence where the hydrogen atom at the end of the corresponding alkane is replaced by the carbon or oxygen atom to which that R so R 1 or R 2 in the diagram above is attached. In this case when naming esters their name would end with the suffix -ate. Esters can have trivial names but in most cases they conform to the IUPAC nomenclature.
So when you encounter a compound in the field that that is a liquid and the name ends in ate there is a good chance it is an ester. No number is assigned to this alkyl chain. Table 153 Nomenclature of Esters.
Esters by the IUPAC naming convention have an -oate ending. In both common and International Union of Pure and Applied Chemistry IUPAC nomenclature the -ic ending of the parent acid is replaced by the suffix -ate Table 153 Nomenclature of Esters. An alkyl group in green is attached directly to the oxygen atom by its middle carbon atom.
For ethers if both parts are different name each one separately using the root plus -yl and end with the word ether. This is followed by the name of the parent chain from the carboxylic acid part of the ester with an e remove and replaced with the ending oate. See Nomenclature and common formula section.
However in this case the IUPAC preferred name is just ethyl acetate instead of ethyl ethanoate. The part derived from the acid that is the benzene ring and the carbonyl group in red is benzoate. Theres no way round this - you just have to get used to it.
-ster names are used more often as masculine names. This is probably the most commonly used example of an ester. In compounds where oxygen breaks a continuous chain of carbons it is named in two parts.
.bmp?revision=1&size=bestfit&width=434&height=321)








