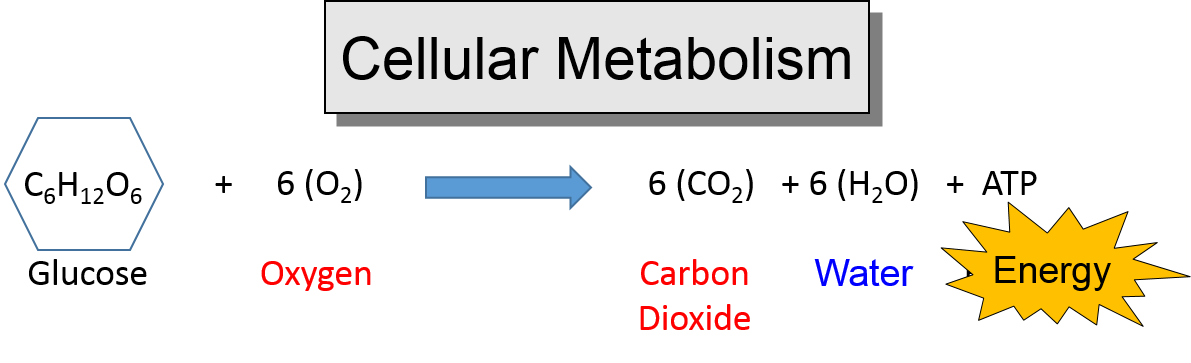
The process by which cells use oxygen to break down food molecules and release their stored energy.
Energy releasing equation. The source of the energy required to regenerate atp is the chemical energy stored in food (e.g. It is the common hydrolysis of atp, in which the nucleophilic oxygen of water attacks the electrophilic central phosphorus of the last. Endothermic reactions absorb energy from the.
The thermochemical reaction can also be written in this way: Atp is adenosine triphosphate, which carries energy in all biological organisms. The total energy released or absorbed is given by :
The energy level of the electron of a hydrogen atom is given by the following. The energy of the electron of a monoelectronic atom depends only on which shell the electron orbits in. The amount of energy exchanged (either absorbed or released) in a chemical reaction is often expressed as a numerical quantity to the right of the equation, labeled δ h, usually defined at a.
Exothermic reactions to a first approximation the. You can get the number of moles in “m” by: Respiration is also referred to as a process involving passage of air and production of.
Enjoying the ease of an energy releasing and balancing with an emotion code practitioner is the simplest way to get rid of your emotional baggage. Some fuels are better than others at releasing energy during combustion reactions. Ch 4 ( g) + 2 o 2 ( g) → co 2 ( g) + 2 h 2 o ( l) δ h = − 890.4 kj.
Echemi.com offers a wide variety of articles about energy releasing equation, easily find your energy releasing equation information here online. The cellular respiration equation is a part of metabolic pathway that breaks down complex carbohydrates. The energy released (sometimes called enthalpy) is calculated using the following equation:








