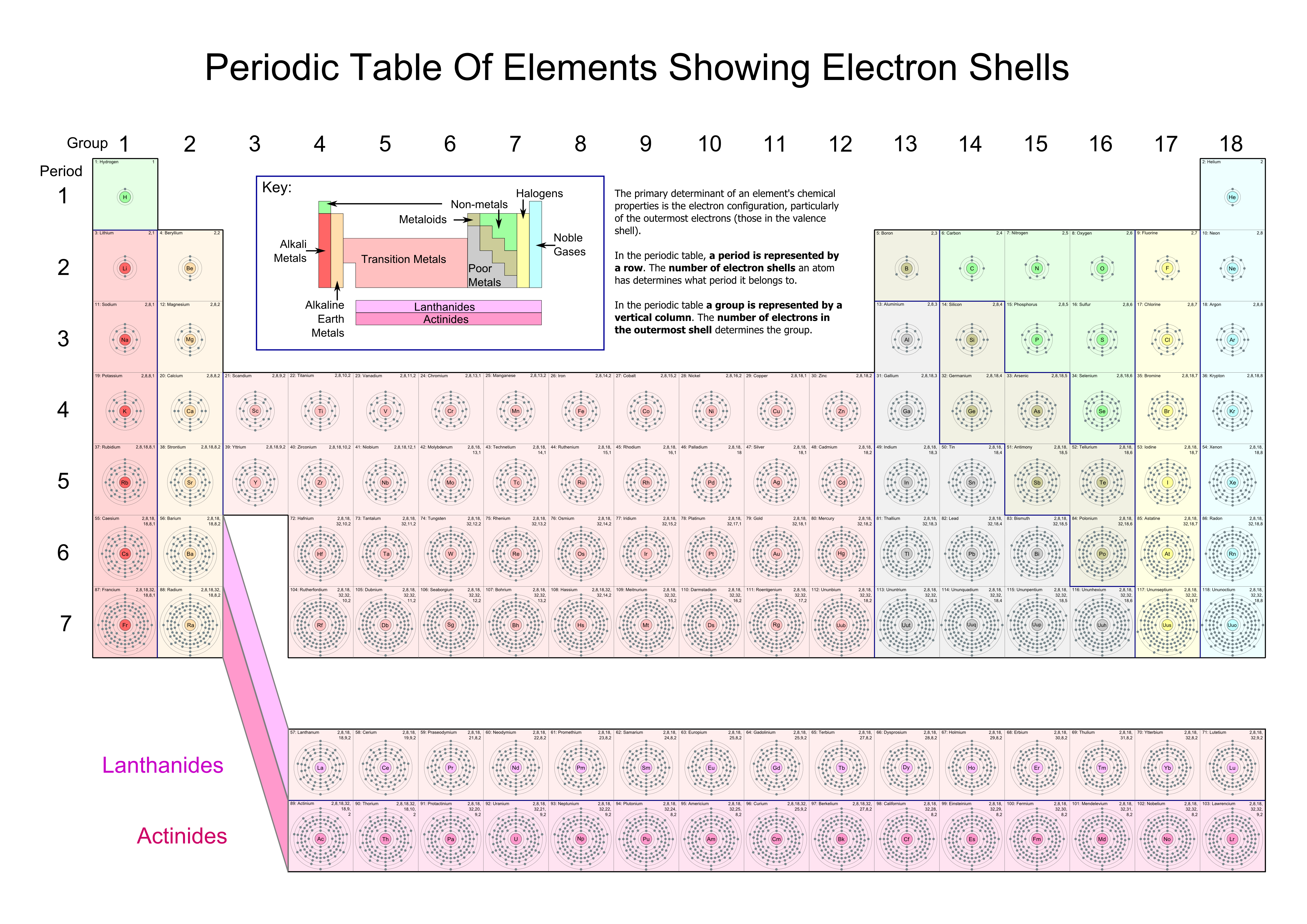
The highest energy level number (1 through 7) for the electrons in an atom corresponds to the period (or row) in the periodic table to which that atom belongs.
Energy levels in periodic table. For instance, lithium () has three electrons: They roughly follow the periods of the periodic table but it. In chemistry, the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atom's nucleus.
Updated on july 14, 2019. The periodic table game available on this page is for entertainment purposes only, and should not be used to grade students on their knowledge of chemical elements. 119 rows chemical elements listed by ionization energy the elements of the periodic table sorted by ionization energy.
After plugging in the values of constants, the equation becomes: The energy levels are depicted on the periodic table and relate to the different orbitals discussed above. Ionization energy of carbon (c) 11.26 ev.
Because there are 7 periods in. The periodic table is an attempt to explain the relationship between different atoms and how they relate to one another to create different forms of energy and matter. Two fill the orbital, and the third is placed in.
N is the principal quantum number (or energy level) of the electron; After the third energy level has 8 electrons (argon), the next 2 electrons go into the fourth energy level. The energy level periodic table is a collection of formulas that help people understand how energy moves about the earth.
The energy of an electron when it is far away from the influence of the nucleus is taken as zero. Elements in the second row of the periodic table place their electrons in the 2n shell as well as the 1n shell. Realiza un listado de 10 elementos de la tabla periódica y ejemplificar gráficamente a cada uno de estos por medio de un objeto que podamos observar.









