Ionization energy of nitrogen (n) 14.53 ev.
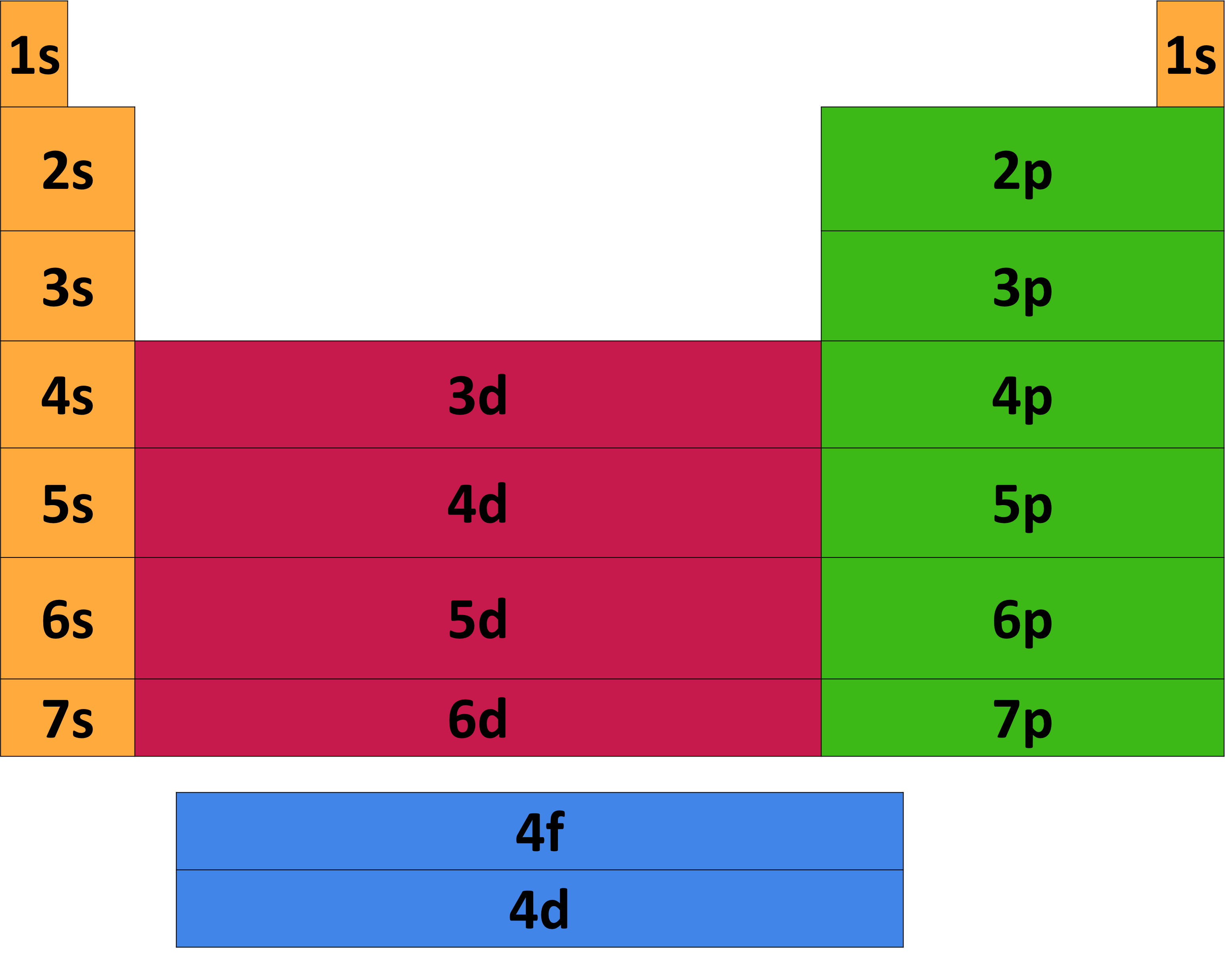
Energy level table. Bohr made the calculation of the energy of an electron in the hydrogen’s atom nth level by considering the electrons in orbits that are circular and quantized. The image map below will direct you to the table of energy levels [pdf format only] for that particular nuclide from the most recent publication found. In these diagrams, energy values are plotted vertically in mev, based.
Select atom and range of upper states : To compute the energies of electrons at the n th level of the hydrogen atom, bohr utilized electrons in circular and quantized orbits. The best kept secrets about unique energy drinks.
The 2s has lower energy when compared to 2p. Find the wavelength of a photon. Ionization energy of carbon (c) 11.26 ev.
Find the wavelength of a photon. Formula of energy of electron. We also give a separate table of energy level data for each.
Electrons in this energy level revolve in the orbit having the smallest possible. The energy level, known popularly as the electron shell, is the orbit with electrons that surround the nucleus of an atom. Sities, the energy levels and transitions probabilities where available are listed for a total of about 2400 lines in these tables.
The energy level periodic table is a collection of formulas that help people understand how energy moves about the earth. Each row of the demand section shows the sum of final demand in one of the top level branches of the demand tree (i.e. Relative to the ground state.








