Hello, in this particular article you will provide several interesting pictures of energy level on periodic table.html. We found many exciting and extraordinary energy level on periodic table.html pictures that can be tips, input and information intended for you. In addition to be able to the energy level on periodic table.html main picture, we also collect some other related images. Find typically the latest and best energy level on periodic table.html images here that many of us get selected from plenty of other images.
 We all hope you can get actually looking for concerning energy level on periodic table.html here. There is usually a large selection involving interesting image ideas that will can provide information in order to you. You can get the pictures here regarding free and save these people to be used because reference material or employed as collection images with regard to personal use. Our imaginative team provides large dimensions images with high image resolution or HD.
We all hope you can get actually looking for concerning energy level on periodic table.html here. There is usually a large selection involving interesting image ideas that will can provide information in order to you. You can get the pictures here regarding free and save these people to be used because reference material or employed as collection images with regard to personal use. Our imaginative team provides large dimensions images with high image resolution or HD.
 energy level on periodic table.html - To discover the image more plainly in this article, you are able to click on the preferred image to look at the photo in its original sizing or in full. A person can also see the energy level on periodic table.html image gallery that we all get prepared to locate the image you are interested in.
energy level on periodic table.html - To discover the image more plainly in this article, you are able to click on the preferred image to look at the photo in its original sizing or in full. A person can also see the energy level on periodic table.html image gallery that we all get prepared to locate the image you are interested in.
 We all provide many pictures associated with energy level on periodic table.html because our site is targeted on articles or articles relevant to energy level on periodic table.html. Please check out our latest article upon the side if a person don't get the energy level on periodic table.html picture you are looking regarding. There are various keywords related in order to and relevant to energy level on periodic table.html below that you can surf our main page or even homepage.
We all provide many pictures associated with energy level on periodic table.html because our site is targeted on articles or articles relevant to energy level on periodic table.html. Please check out our latest article upon the side if a person don't get the energy level on periodic table.html picture you are looking regarding. There are various keywords related in order to and relevant to energy level on periodic table.html below that you can surf our main page or even homepage.
 Hopefully you discover the image you happen to be looking for and all of us hope you want the energy level on periodic table.html images which can be here, therefore that maybe they may be a great inspiration or ideas throughout the future.
Hopefully you discover the image you happen to be looking for and all of us hope you want the energy level on periodic table.html images which can be here, therefore that maybe they may be a great inspiration or ideas throughout the future.
 All energy level on periodic table.html images that we provide in this article are usually sourced from the net, so if you get images with copyright concerns, please send your record on the contact webpage. Likewise with problematic or perhaps damaged image links or perhaps images that don't seem, then you could report this also. We certainly have provided a type for you to fill in.
All energy level on periodic table.html images that we provide in this article are usually sourced from the net, so if you get images with copyright concerns, please send your record on the contact webpage. Likewise with problematic or perhaps damaged image links or perhaps images that don't seem, then you could report this also. We certainly have provided a type for you to fill in.

 Labeled Periodic Table Energy Levels - Periodic Table Timeline
Labeled Periodic Table Energy Levels - Periodic Table Timeline
 Electron Energy Levels Periodic Table - Periodic Table Timeline
Electron Energy Levels Periodic Table - Periodic Table Timeline
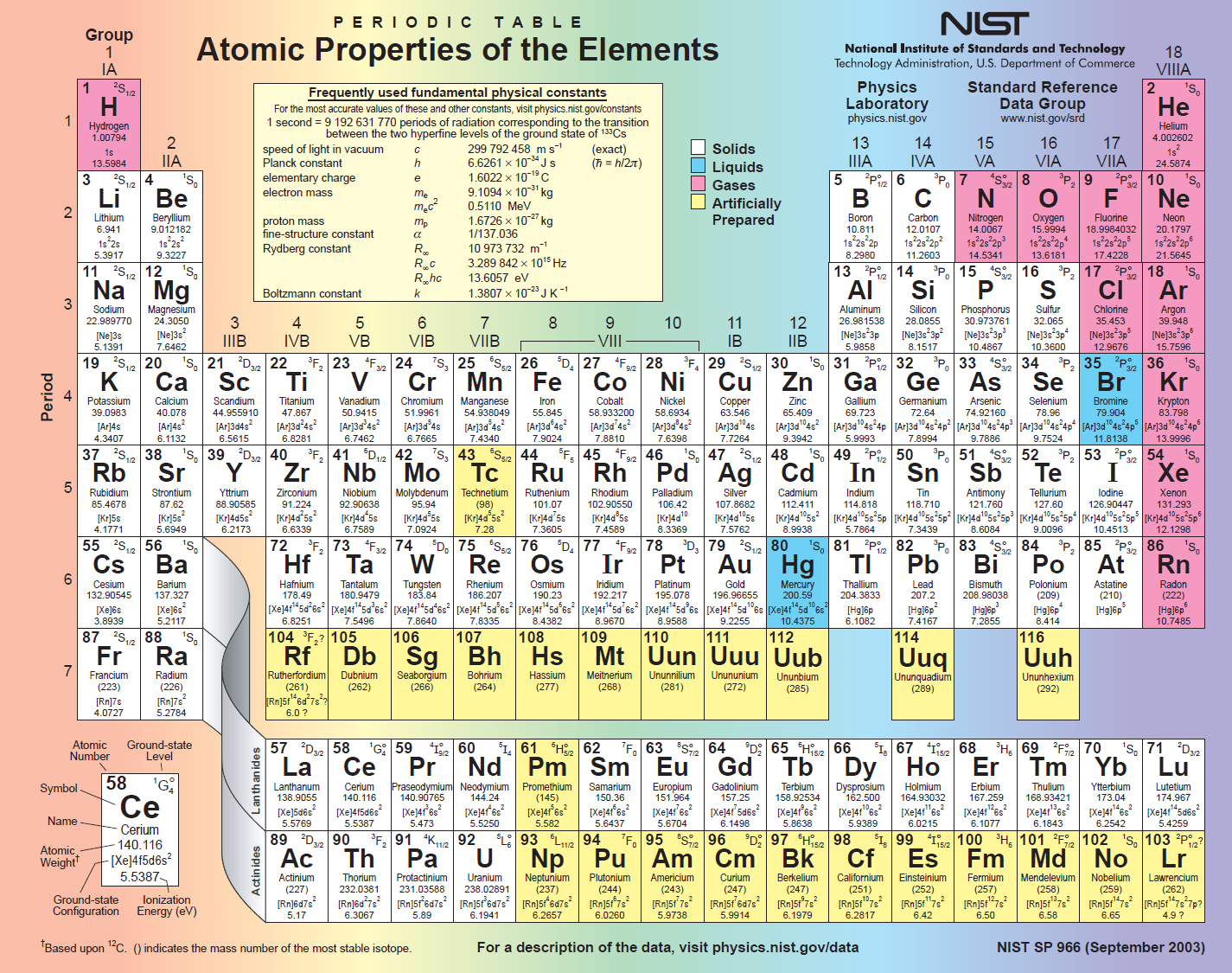 Periodic Table
Periodic Table
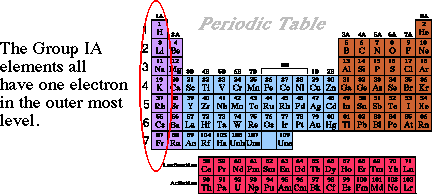 Electron Energy Levels Periodic Table - Periodic Table Timeline
Electron Energy Levels Periodic Table - Periodic Table Timeline
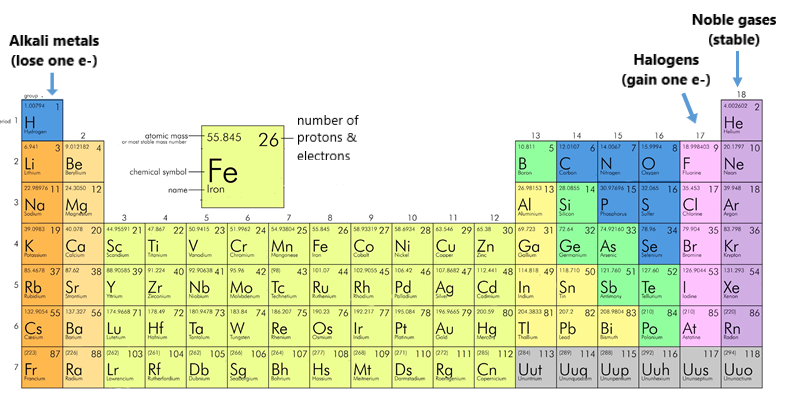
 Periodic Table - MVHS CHEMISTRY
Periodic Table - MVHS CHEMISTRY
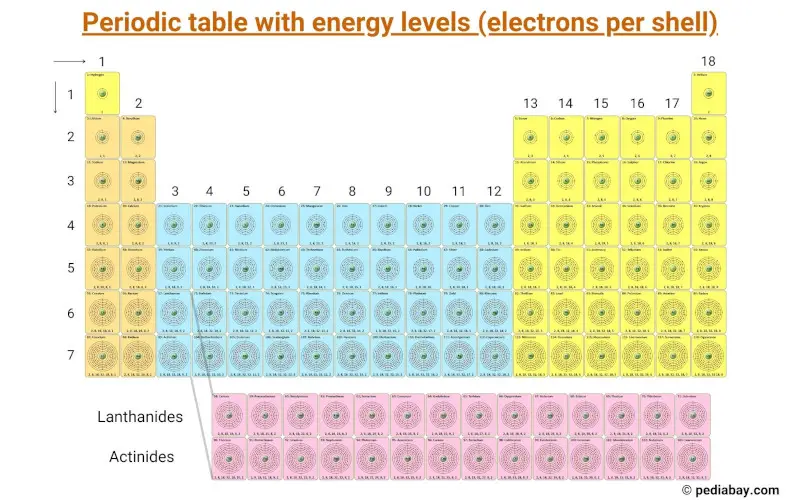
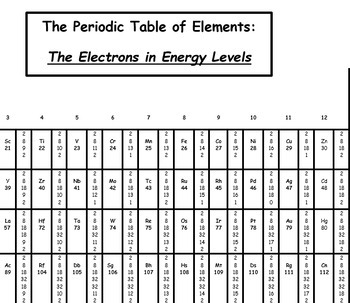 Periodic Table With Energy Levels Shown - Periodic Table Timeline
Periodic Table With Energy Levels Shown - Periodic Table Timeline
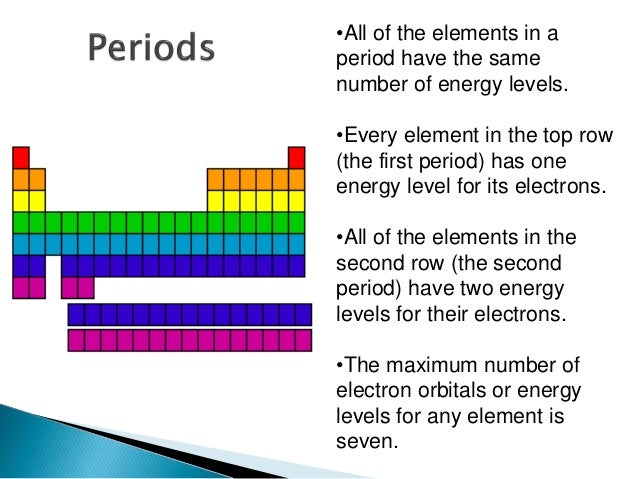 Periodic Table Of Elements Energy Levels - Periodic Table Timeline
Periodic Table Of Elements Energy Levels - Periodic Table Timeline
 Periodic Table - MVHS CHEMISTRY
Periodic Table - MVHS CHEMISTRY
 Spdf Periodic Table Energy Levels - Periodic Table Timeline
Spdf Periodic Table Energy Levels - Periodic Table Timeline
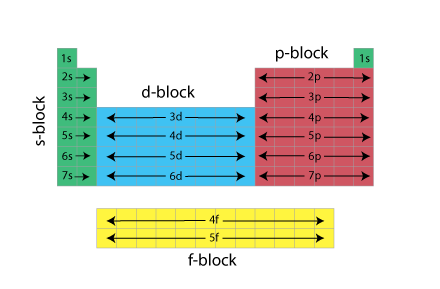 Periodic Table Of Elements Energy Levels - Periodic Table Timeline
Periodic Table Of Elements Energy Levels - Periodic Table Timeline
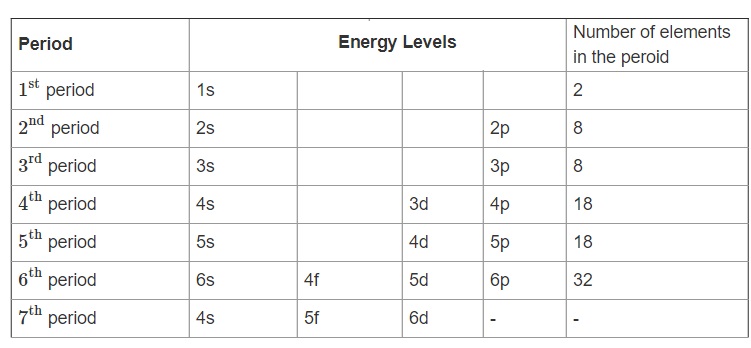 Electron Configuration Periodic Table Energy Levels - Periodic Table
Electron Configuration Periodic Table Energy Levels - Periodic Table
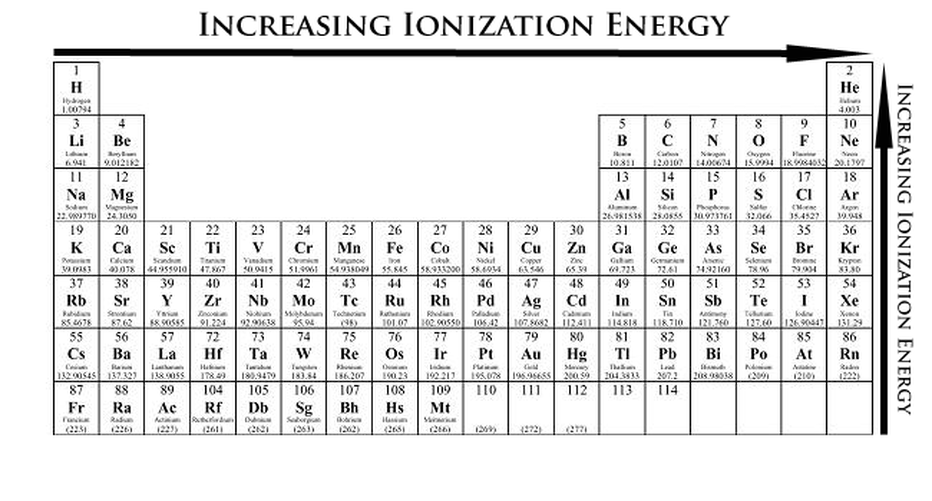
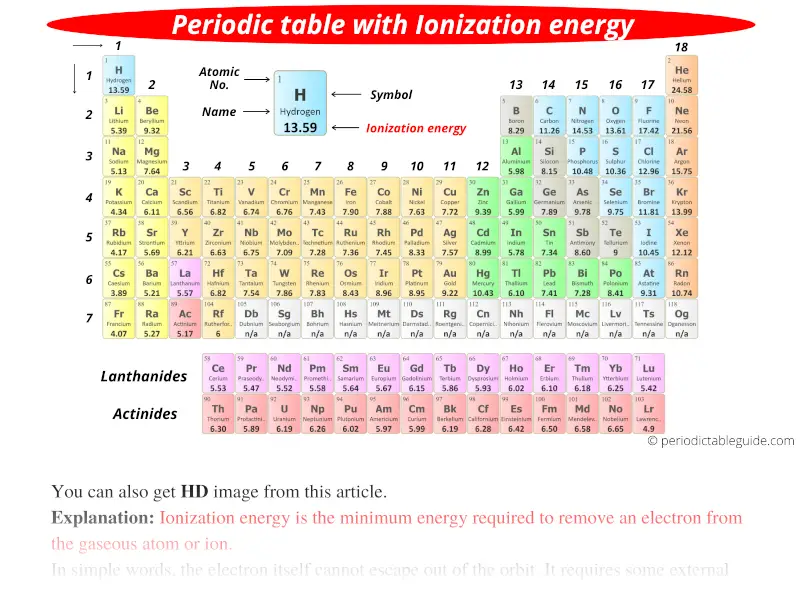 Periodic table with Ionization Energy Values (Labeled Image)
Periodic table with Ionization Energy Values (Labeled Image)
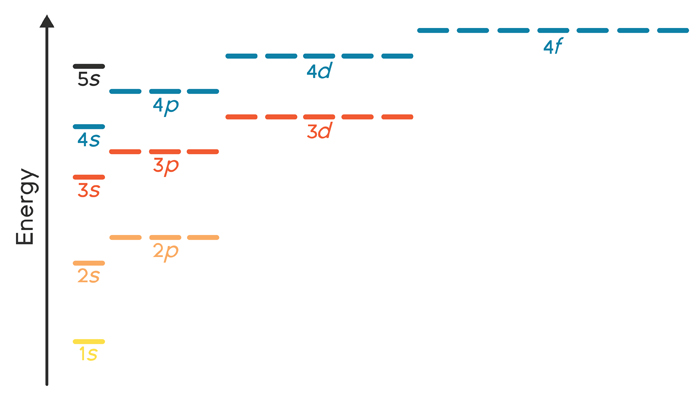 Labeled Periodic Table Energy Levels - Periodic Table Timeline
Labeled Periodic Table Energy Levels - Periodic Table Timeline
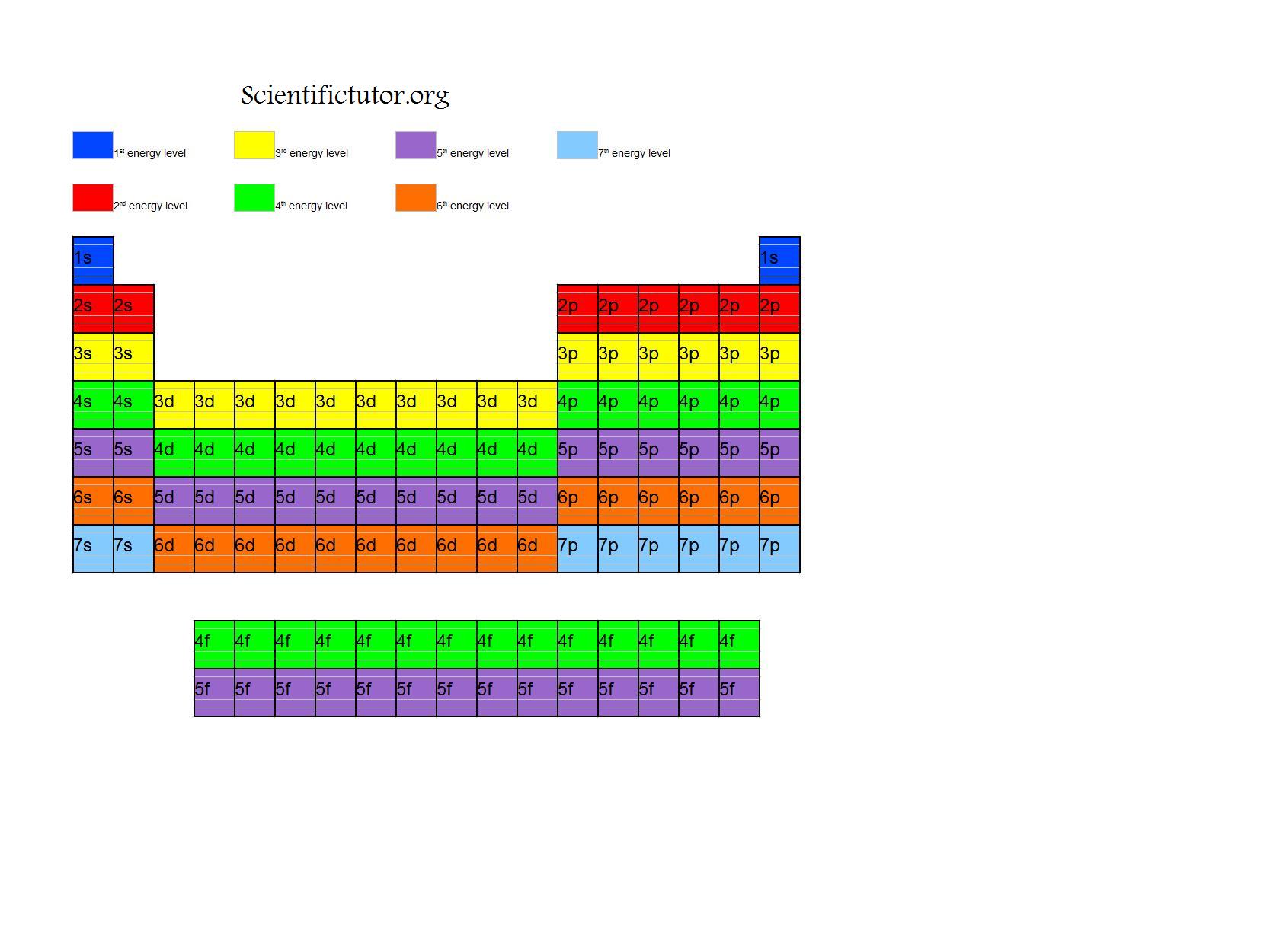
 The Periodic Table by Energy Levels
The Periodic Table by Energy Levels
.PNG)