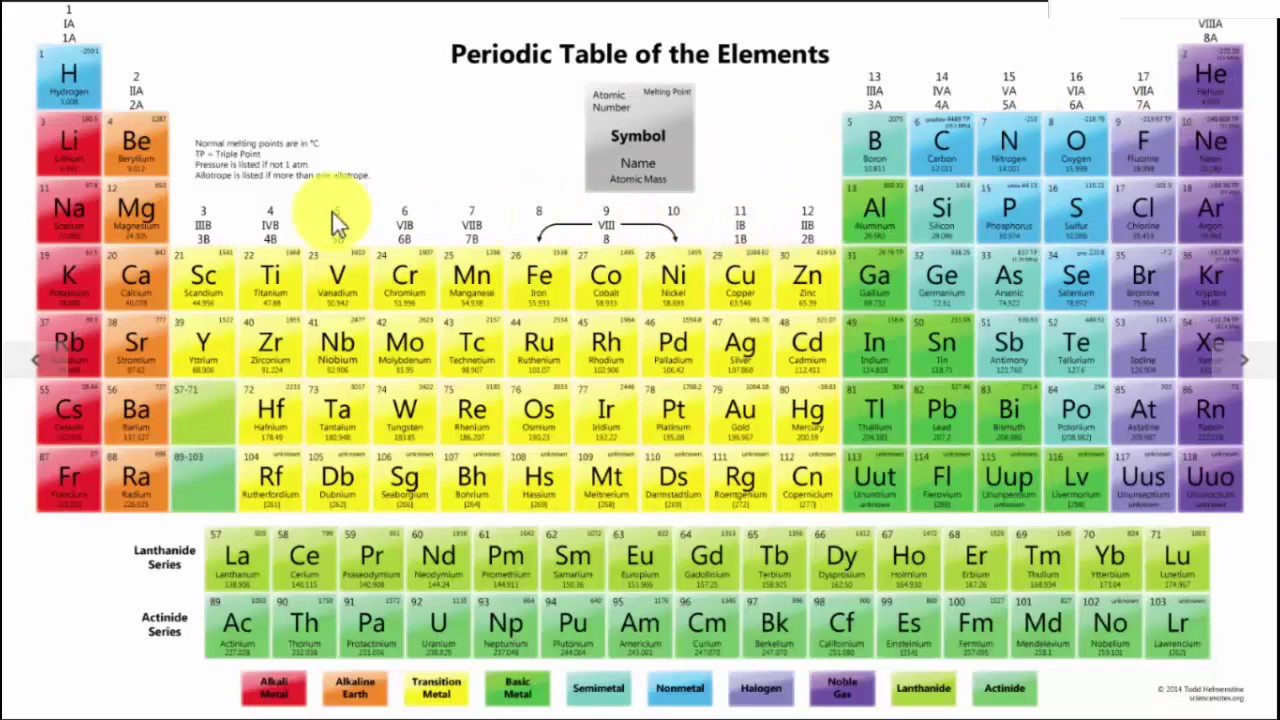
The periodic table, also known as the periodic table of the (chemical) elements, is a tabular display of the chemical elements.it is widely used in chemistry, physics, and other sciences,.
Elements are arranged by. The arrangement of elements in the periodic table starts from the very first top left. The periodic table elements are arranged in the increasing order of their atomic number. Newlands proposed classifying the elements in the order of increasing atomic weights, the elements being assigned ordinal numbers from unity upward.
There are 8 different groups: Lithium (li), sodium (na), potassium (k), rubidium (rb), cesium (cs), and francium (fr). Lithium, neon and the elements between them are in period 2;
Group 2 is for alkaline earth. Chemists typically place elements in order of increasing atomic numbers in a. Group 1 is for alkali metals:
Periodic table is organized by their valence electrons, atomic number and their atomic mass ( and also their reactivity/ groups and families). Let me show you how they are arranged… as shown in the above image,. Hydrogen and helium are in period 1;
All the elements of the periodic table are arranged in order of increasing atomic (proton) number. How are elements arranged in the periodic table? The vertical columns are called groups.
The elements are also arranged horizontally in periods. Today, 150 years later, chemists officially recognize 118 elements (after the addition of four newcomers in 2016) and still use mendeleev's periodic table of elements to organize. The elements are also arranged horizontally in periods.









