It can be represented as:
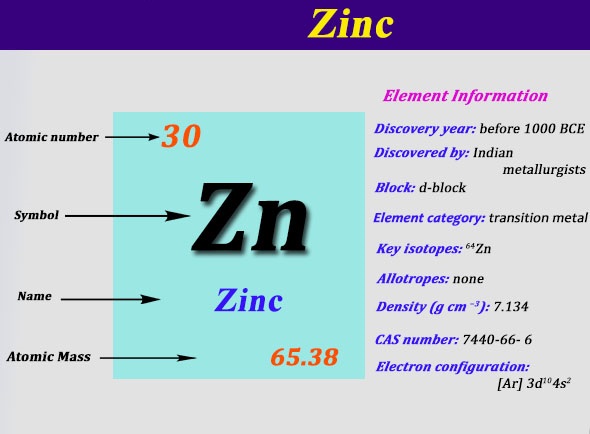
Electronic configuration of zn. The zinc atom donates two electrons in the 4s orbital to form a zinc ion(zn 2+). 1s2 2s2 2p6 3s2 3p6 3d10 4s2. Electronic configuration of zinc zn:
Electron configuration of helium (he) 1s 2: The zn 2+ ion has lost two electrons, which leaves it with 30 protons and 28 electrons. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury.
Nevertheless, check the complete configuration and other interesting facts about zinc that. Zinc ion (zn 2+) electron configuration. What is the electronic configuration of chlorine 17?
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 14 6d 10 7p 6 alternatively, write the symbol for the noble gas. [ ar] 3 d 10 4 s 2 The zinc atom exhibits a zn 2+ ion.
It’s electron configuration would be represented as 1s2 2s2 2p6 3s2 3p6 4s2 3d10; Five stable isotopes of zinc occur in nature, with 64 zn being the most abundant isotope (49.17% natural abundance). Zinc (zn), atomic # 30, is located in the “d” block in period 4;
In the case of zinc the abbreviated electron configuration is [ar] 3d10 4s2. 5 rows zinc ion(zn 2+) electron configuration. The electronic configuration of zinc in the ground state is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2.







