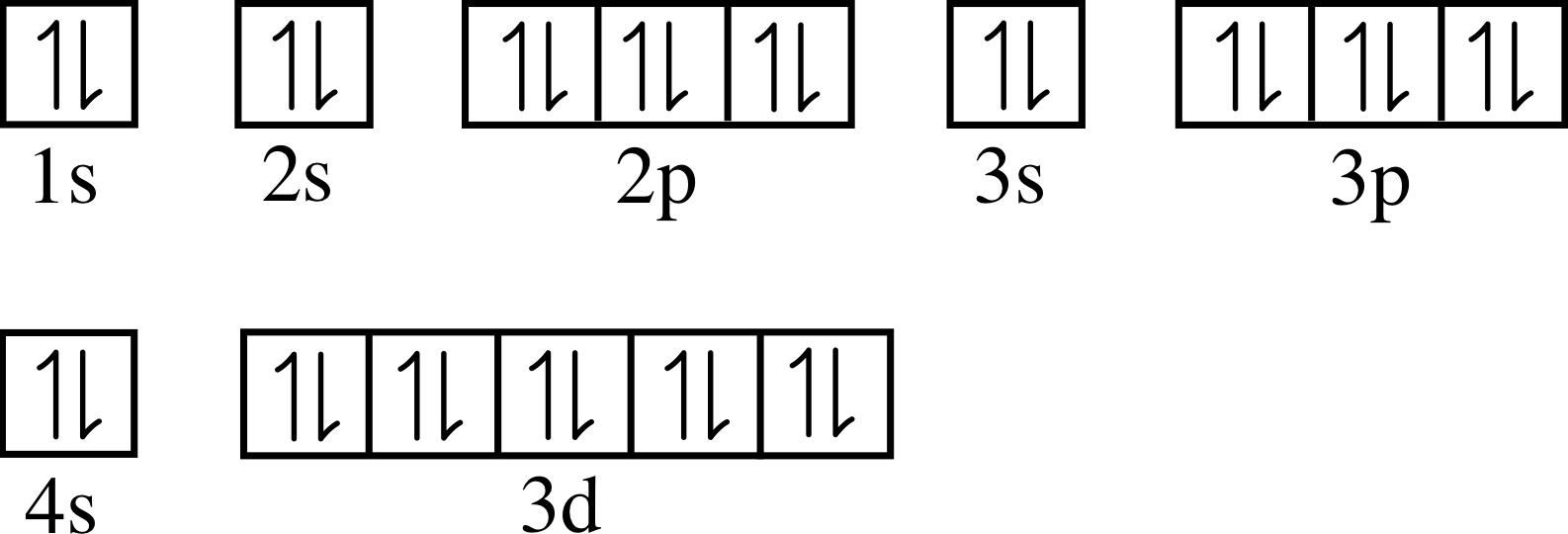
1s2 2s2 2p6 3s2 3p6 3d10 4s2.
Electronic configuration of zinc. Full electron configuration of zinc: To find the electron dot diagram for zinc, we need to write its electronic configuration according to aufbau’s principle. 119 rows the electronic configuration of each element is decided by the aufbau principle which states that the electrons fill orbitals in order of increasing energy levels.
The electronic configuration of chromium is 1s2 ,2s2 , 2p6 , 3s2 ,3p6 ,4s1 ,3d5 and not 1s2 ,2s2 , 2p6 , 3s2 ,3p6 ,4s2 ,3d4. The chemical symbol of zinc is 'zn'. The full electron configuration of zinc is 1s2 2s22p6 3s23p63d10 4s2zinc, also written zinc, is defined as the chemical element that belongs to the periodic table of elements.
Electron configuration of zinc (2, 8, 18, 2) atomic number: Ar 3d10 4s2 ad 3d10 4s2 ar 3d11 4s2 none of these get the answers you need, now! The 2+ charge denotes that zinc has loosened 2 electrons from its valence shell.
The atomic number of zinc is 30, so 30 electrons are present in its neutral state, so configuration in. Zn2+ = 1s2 2s2 2p6. In general, the electronic configuration of these.
119 rows electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas configuration) as well as full. Copper ← zinc → gallium. What is the electronic configuration of zinc?
It is a moderately reactive metal and strong reducing agent. Zinc (zn) [ar] 3d 10 4s 2: Electronic configuration of the zinc atom in.








