Located in the iv period.
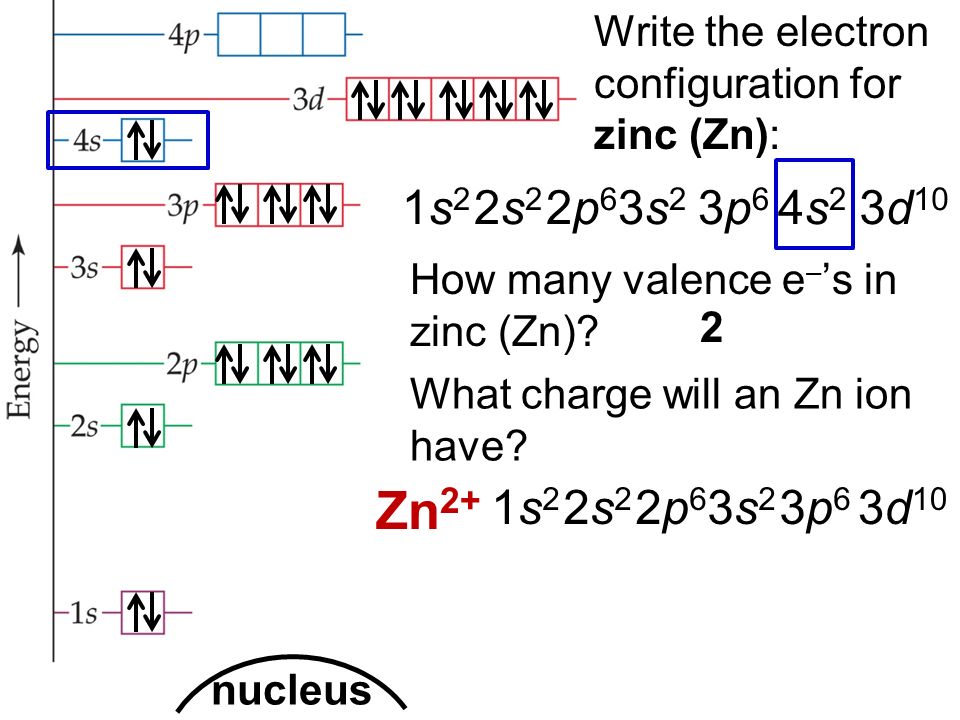
Electronic configuration of copper and zinc. Elements like mercury and zinc are not considered transition metals because they have electronic configurations: Copper influences the electrical and optical properties of zinc oxide to a high degree. 1s2 2s2 2p6 3s2 3p6 3d10 4s1.
1s2 2s2 2p6 3s2 3p6 3d10 4s2. Electronic configuration of copper is [kr] 3d 10 4s 1 and electronic configuration of zinc is [kr] 3d 10 4s 2. At zinc the shell is full with [ar] 4s2 3d10.
Located in the iv period. The first ionization potential of zinc is higehr than cu. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.
Full electron configuration of copper: This give us the (correct) configuration of: Electronic configuration of the zinc atom in.
Complete step by step answer: Atomic number of cu = 2 9. Previously this has been explained within the frame of a model assuming cu++ ions to be substitutional.
The electronic configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 10. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 1 0.









